Apremilast: A Review in Psoriasis and Psoriatic Arthritis
General Description
Apremilast, also known as Otezla, is a phosphodiesterase 4 (PDE4) inhibitor used to treat various types of symptoms resulting from certain inflammatory autoimmune diseases. It belongs to the same drug class as Roflumilast and Crisaborole. Initially approved in 2014, it is marketed by Celgene. In July 2019, apremilast was granted a new FDA approval for the treatment of oral ulcers associated with Behcet's disease, an autoimmune condition that causes recurrent skin, blood vessel, and central nervous system inflammation.1-3

Figure 1. Properties of Apremilast Tablets
Therapeutic Efficacies
Apremilast 30 mg twice daily was effective in the treatment of moderate to severe plaque psoriasis [23, 24]. In both ESTEEM 1 and ESTEEM 2, the PASI-75 response rate and the sPGA response rate were significantly higher with apremilast than with placebo at week 16. Apremilast improved nail and scalp psoriasis, according to subgroup analyses of ESTEEM 1 and ESTEEM 2 that included patients with nail psoriasis [Nail Psoriasis Severity Index (NAPSI) score of C1] or moderate to very severe scalp psoriasis [Scalp Physician Global Assessment (ScPGA) score of C3].5-6
Apremilast improved signs and symptoms in DMARD experienced and DMARD-naive patients with active psoriatic arthritis. Apremilast monotherapy had an early onset of efficacy, with a significantly higher ACR20 response rate seen in patients receiving apremilast than in those receiving placebo at week 2 (16.4 vs. 6.4%; p = 0.0252).7-9
Mechanism of Action
Apremilast is an orally administered, small molecule inhibitor that is selective for PDE4. Inhibition of PDE4 by apremilast prevents cAMP being hydrolysed toAMP, resulting in increased intracellular cAMP levels and downstream effects on multiple intracellular signalling pathways in various cell types, including innate immune cells (e.g. monocytes), adaptive immune cells (e.g. T cells) and non-immune cells (e.g. keratinocytes, synovial fibroblasts). Apremilast also leads to the activation of cyclic nucleotide-gated ion channels and Epac 1/2.In vitro, apremilast inhibited the expression and/or production of pro-inflammatory cytokines and chemokines from various cell types. Apremilast increased the production of IL-10 in stimulated PBMCs [6, 9]. Apremilast demonstrated activity in murine models of psoriasis and arthritis.10
Pharmacodynamics
In patients with moderate to severe plaque psoriasis, significantly (p < 0.01) greater median percentage reductions from baseline to week 4 in plasma levels of IL-17A, IL-17F, IL-22 and TNF-a were seen in those receiving monotherapy with the recommended dosage of apremilast 30 mg twice daily (n = 83) than in those receiving placebo (n = 47), according to a substudy of the phase 3 ESTEEM2 trial. In addition, in a substudy of a phase 2b Japanese trial in patients with moderate to severe plaque psoriasis, significantly (p < 0.01) greater median percentage reductions from baseline to week 4 in plasma levels of IL-17A, IL-17F and IL-22 were seen in those receiving apremilast 30 mg twice daily (n = 22) than in those receiving placebo (n = 23). In ESTEEM-2, a significant (p < 0.001) correlation was seen between the percentage change from baseline to week 4 in IL-17A, IL-17F and IL-22 levels and the percentage improvement from baseline to week 16 in the Psoriasis Area and Severity Index (PASI) score. Further analysis revealed that in patients with psoriasis, IL-17F is the most important predictor of PASI response to apremilast, and that synergistic cytokine effects seen at week 4 predicted PASI improvement with apremilast at week 16.10
Pharmacokinetics
Oral apremilast has an absolute bioavailability of ~73%. The maximum plasma concentration (Cmax) of apremilast was reached in a median of ~2.5 h; apremilast may be administered without regard to food. Apremilast is ~68% plasma protein bound, with a mean apparent volume of distribution of 87 LApremilast undergoes extensive metabolism via both CYP-mediated oxidative metabolism (with subsequent glucuronidation) and non-CYP mediated hydrolysis. CYP3A4 was primarily responsible for CYPmediated metabolism of apremilast in vitro, with minor contributions from CYP1A2 and CYP2A6. Following administration of radiolabelled apremilast, ~58 and 39% of radioactivity was recovered in urine and faeces, with ~3 and 7% of the dose recovered in the urine and faeces as the parent drug. Plasma clearance of apremilast was ~10 L/h with a terminal elimination half-life of ~6–9 h.11
Safety and Tolerability
Apremilast was generally well-tolerated with an acceptable safety profile among patients in clinical trials. A long-term, pooled safety analysis monitored 1250 patients (832 treated with apremilast, 418 treated with placebo) in the ESTEEM trials for more than 156 weeks for treatment-related adverse events (AEs). The most common reasons for discontinuing the study were lack of efficacy (34.7%) and patient withdrawal from the study (18.5%). Most AEs presented within the first week of dosing and self-resolved within one month. AE rates decreased over time and did not increase with longer exposure to apremilast treatment. Few patients discontinued apremilast therapy due to AEs.12
The most commonly reported adverse events (AEs) were diarrhea (n = 221, 18.7%), nausea (n = 195, 16.5%), upper respiratory tract infection (n = 227, 19.2%), nasopharyngitis (n = 196, 16.6%), and headache (n = 201, 17.0%). Severe and serious AEs were uncommon and comparable between treatment and placebo groups, occurring in around 2% of patients in each group in both trials. Secretory diarrhea, nausea, and vomiting were thought to be induced by PDE4 inhibition; a similar mechanism is implicated in caffeine-induced diarrhea.12
Synthesis
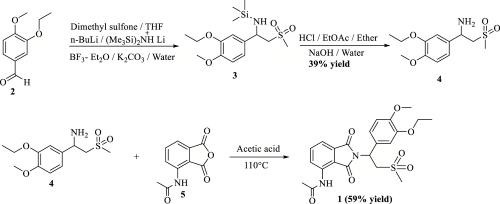
Figure 2. Synthesis of Apremilast
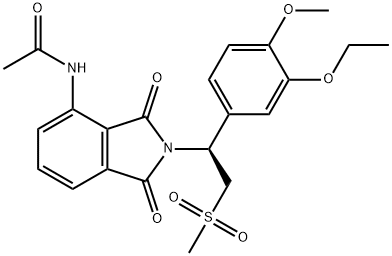
The synthesis route of racemic apremilast was first reported by Celgene Corporation in the United States. The first total synthesis of racemic apremilast, which was described in U.S. Patent 6,020,358, initiated the synthesis using 3-ethoxy-4-methoxybenzaldehyde (2). In first step, dimethyl sulfone reacts with n-butyllithium to obtain lithium dimethyl sulfone; this was added to the mixture of 3-ethoxy-4- methoxybenzaldehyde (2), lithium hexamethyldisilazide, and boron trifluoride etherate to obtain 1-(3-ethoxy-4-methoxyphenyl)-2-(methyl sulfonyl) ethan-1-amine (4) in 39% yield. This was further condensed with 3-acetamido phthalic anhydride (5) in acetic acid at 110 °C to afford apremilast (1) in 59% yield. The synthesis of racemic amine 4 has been reported in many research groups using different starting material.13
References
1. Afra TP, Razmi TM, Dogra S: Apremilast in Psoriasis and Beyond: Big Hopes on a Small Molecule. Indian Dermatol Online J. 2019 Jan-Feb;10(1):1-12.
2. FDA Approved Drug Products: Otezla (apremilast) tablets for oral use.
3. Suzuki Kurokawa M, Suzuki N: Behcet's disease. Clin Exp Med. 2004 Sep;4(1):10-20.
4. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
5. Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99.
6. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–6.
7. Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43(9):1724–34.
8. Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75(6):1065–73.
9. Wells A, Adebajo AO, Aelion JA, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52- week) improvement in the signs and symptoms of psoriatic arthritis in DMARD-naive patients: results from a phase 3, randomized, controlled trial [abstract no. 1543]. Arthritis Rheumatol. 2014;66(Suppl 10):S680.
10. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–29.
11. Celgene Corporation. Otezla (apremilast) tablets, for oral use: US prescribing information. 2015. http://www.celgene.com/ content/uploads/otezla-pi.pdf. Accessed 14 Feb 2023.
12. Crowley J, Tha?i D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(2):310–317.e311.
13. Narode, H., Gayke, M., Eppa, G., & Yadav, J. S. (2021). A review on synthetic advances toward the synthesis of apremilast, an anti-inflammatory drug. Organic Process Research & Development, 25(7), 1512-1523.
);Related articles And Qustion
See also
Lastest Price from Apremilast manufacturers

US $10.00/kg2024-04-22
- CAS:
- 608141-41-9
- Min. Order:
- 1kg
- Purity:
- 99.7%
- Supply Ability:
- 200000kg

US $0.00/Kg/Bag2024-04-22
- CAS:
- 608141-41-9
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up
- Supply Ability:
- 20 tons