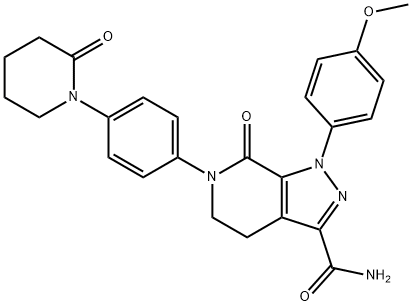
Apixaban: mechanism of action, pharmacokinetics, pharmacodynamic and applications
General Description
Apixaban is a potent and selective inhibitor of factor Xa, with strong antithrombotic activity. It inhibits thrombus formation by targeting free and clot-bound FXa as well as prothrombinase activity. The drug reduces thrombin generation, inhibits clot growth, and indirectly suppresses platelet aggregation induced by thrombin. Apixaban's absorption primarily occurs in the small intestine, and its bioavailability is approximately 50%, due to incomplete absorption and first-pass metabolism. Clinically, it is used to prevent stroke and systemic embolism in non-valvular atrial fibrillation, treat and prevent venous thromboembolism, provide post-operative venous thromboembolism (VTE) prophylaxis, and secondary prevention in acute coronary syndrome and myocardial infarction patients. Individualized dosing and duration should be determined by healthcare professionals based on patient factors and medical history.
Figure 1. Tablets of apixaban
Mechanism of action
Apixaban is a potent, direct, oral, reversible, and highly selective inhibitor of FXa (inhibitory constant = 0.08 nM [0.037 ng/mL] at 25°C) that does not require antithrombin III for antithrombotic activity. Apixaban inhibits free and clot-bound FXa, as well as prothrombinase activity, which inhibits clot growth. By inhibiting FXa, apixaban decreases thrombin generation and thrombus development. It has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. In a rabbit arteriovenous shunt thrombosis model, apixaban inhibited thrombus formation in a dose-dependent manner (half maximal inhibitory concentration = 329 nM). 1
Pharmacokinetics
The maximum plasma concentration (Cmax) of apixaban occurs 3–4 h after oral administration. The absorption of apixaban appears to occur primarily in the small intestine and decreases progressively throughout the gastrointestinal tract. Compared with oral administration, the bioavailability of 2.5 mg of apixaban solution was approximately 60% and 84% lower when released in the distal small bowel and ascending colon, respectively. For oral doses up to 10 mg, the absolute bioavailability of apixaban is 50%, resulting from the incomplete absorption and first-pass metabolism in the gut and liver. 2
Pharmacodynamic
The pharmacodynamic effects of apixaban observed in clinical studies were consistent with its mechanism of action: direct reversible inhibition of FXa. The relationships between apixaban plasma concentrations and clotting time measures as well as anti-FXa activity are illustrated. Apixaban prolongs traditional clotting tests such as prothrombin time, international normalized ratio, and activated partial thromboplastin time. Clotting times showed dose-related increases tracking the plasma concentration–time profile; however, changes are small, subject to a high degree of variability, and not useful in monitoring the anticoagulant effect of apixaban. The modified prothrombin time assay was developed as an exploratory pharmacodynamic measure of apixaban to improve the dynamic range of the prothrombin time assay. As expected, the modified prothrombin time results in healthy subjects showed a more sensitive assessment of apixaban activity than international normalized ratio or activated partial thromboplastin time. 3
Applications
Apixaban is widely used in clinical practice for various purposes. One of its primary applications is for the prevention of stroke and systemic embolism in individuals with non-valvular atrial fibrillation. This condition increases the risk of blood clot formation in the heart, which can lead to severe complications like stroke or systemic embolism. By inhibiting the clotting process, apixaban helps reduce the occurrence of these adverse events. Another important use of apixaban is in the treatment and prevention of VTE, which includes deep vein thrombosis (DVT) and pulmonary embolism (PE). It serves as both a therapeutic agent for existing cases of DVT or PE, as well as a prophylactic measure to prevent their recurrence. By targeting factor Xa, apixaban helps prevent the formation of new blood clots and the extension of existing ones, thereby reducing the potential harm caused by VTE. Apixaban is also commonly used for post-operative VTE prophylaxis in orthopedic surgery, particularly in hip or knee replacement procedures. These surgeries pose an increased risk of VTE due to immobilization and trauma to blood vessels. Apixaban is prescribed during the post-operative period to mitigate the risk of blood clot formation and its associated complications. Furthermore, apixaban may be employed as a secondary prevention strategy for patients with acute coronary syndrome or a history of myocardial infarction. When used alongside antiplatelet therapy, apixaban reduces the likelihood of recurrent cardiovascular events. Its anticoagulant properties help prevent the formation of clots that could potentially block narrowed arteries and cause further damage to the heart. 4
Reference
1. Byon W, Garonzik S, Boyd RA, Frost CE.
Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review. Clin
Pharmacokinet, 2019, 58(10):1265-1279.
2 Byon W, Nepal S, Schuster AE, Shenker A, Frost CE. Regional gastrointestinal absorption of apixaban in healthy subjects. J Clin Pharmacol, 018, (7):965–971.
3. Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, et al. Apixaban, an oral, direct factor Xa inhibitor: single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol, 2013, 75(2):476–487.
4. Farge D, Frere C, Connors JM, et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol, 2022, 23(7):e334-e347.
);You may like
Related articles And Qustion
See also
Lastest Price from Apixaban manufacturers

US $50.00-30.00/kg2024-04-30
- CAS:
- 503612-47-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 1000

US $15.00-1.00/kg2024-04-30
- CAS:
- 503612-47-3
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 500