Antioxidant 1024: Synthesis Method and Applications in Nanocomposites
General Description
Antioxidant 1024, synthesized with a high yield of 99%, significantly enhances the thermo-oxidative stability of PP/PP-g-MA/OMMT nanocomposites. Its presence delays degradation reactions, improving overall stability parameters. Research shows that Antioxidant 1024, when paired with a metal deactivator, positively impacts the thermal stability of these nanocomposites. By analyzing the effects of a compatibilizing agent and Antioxidant 1024 through various techniques, it is evident that the antioxidant delays the onset of exothermal reactions, directly linked to its concentration. Increasing the stabilizer concentration leads to controlled and delayed degradation reactions, enhancing the nanocomposites' stability. Overall, the incorporation of Antioxidant 1024 proves crucial in enhancing the performance and longevity of PP/PP-g-MA/OMMT nanocomposite materials by effectively improving their thermo-oxidative stability.

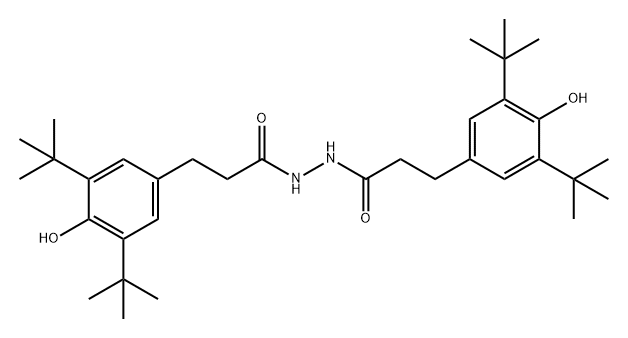
Figure 1. Antioxidant 1024
Synthesis Method
Antioxidant 1024, also known as N,N-bis(β-(3,5-di-t-butyl-4-hydroxybenzyl)acetyl)hydrazine, is synthesized through a specific method to ensure a high yield of 99%. The process involves several steps and the use of different solvents. Firstly, the reaction starts with hydrazine hydrate and β-(3,5-di-t-butyl-4-hydroxybenzyl)propionic acid methyl ester. These two compounds react together to form β-(3,5-di-t-butyl-4-hydroxybenzyl)propionic hydrazide. The reaction takes place in the presence of methanol, toluene, and water as solvents. The reaction conditions are set at a temperature of 85 °C for a duration of 3 hours. Once the β-(3,5-di-t-butyl-4-hydroxybenzyl)propionic hydrazide is obtained, it undergoes another reaction with ethyl acetylacetate. This subsequent reaction leads to the formation of the final product, antioxidant 1024. To achieve a high yield, several factors are carefully controlled during the synthesis process. These include the choice of solvents in the reaction system, the mass ratio of reactants, and the mass/volume ratio of reactants and solvent. By optimizing these parameters, the process ensures that the desired product is obtained with a yield of 99%. In conclusion, the synthesis of antioxidant 1024 involves the reaction of hydrazine hydrate with β-(3,5-di-t-butyl-4-hydroxybenzyl)propionic acid Me ester, followed by a subsequent reaction with ethyl acetylacetate. The process is carried out using specific solvents and carefully controlled conditions to achieve a high yield of 99%. 1
Applications in Nanocomposites
Antioxidant 1024 plays a crucial role in enhancing the thermo-oxidative stability of polypropylene/polypropylene-grafted maleic anhydride/organic montmorillonite (PP/PP-g-MA/OMMT) nanocomposites. In the study mentioned, the researchers focused on understanding how the presence of a metal deactivator bonded to a primary stabilizer like Antioxidant 1024 influences the thermal stability of these nanocomposites. The research involved analyzing the effect of a compatibilizing agent and Antioxidant 1024 on the degradation behavior of the nanocomposites using various techniques such as TGA, DSC, OIT, and dynamic OIT. The results indicated that the use of compatibilizing agents initially improved the stability of the nanocomposites during the early stages of degradation. However, at higher temperatures, there was a decrease in Tmax, suggesting a degradative effect of the compatibilizing agents. On the other hand, the addition of Irganox MD 1024 resulted in notable improvements across all parameters assessing the stability of the nanocomposites. Most significantly, the presence of Antioxidant 1024 delayed the onset of exothermal reactions to higher temperatures. This delay in the initiation of exothermic reactions is directly related to the concentration of the antioxidant, showcasing its effectiveness in enhancing the thermal stability of the nanocomposites. Furthermore, the study discussed how the concentration of Antioxidant 1024 impacted Tmax and the temperature difference between the onset of heat release and initial weight loss. As the concentration of the stabilizer increased, this temperature difference decreased, indicating a more controlled and delayed onset of degradation reactions. In conclusion, the incorporation of antioxidant 1024, in PP/PP-g-MA/OMMT nanocomposites proves to be instrumental in improving their thermo-oxidative stability. By effectively delaying the onset of degradation reactions and enhancing overall stability parameters, this antioxidant plays a vital role in enhancing the performance and longevity of these nanocomposite materials. 2
Reference
1. Guo, JY. Method for synthesizing antioxidant 1024. 2018. Patent Number: CN108484434.
2. Fitaroni LB, de Lima JA, Cruz SA, Waldman W. Effect of compatibilizer and Irganox MD 1024 on the thermo-oxidative stability of PP/PP-g-MA/OMMT nanocomposites.
Related articles And Qustion
See also
Lastest Price from antioxidant 1024 manufacturers

US $0.00/KG2025-09-18
- CAS:
- 32687-78-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $0.00/KG2025-04-21
- CAS:
- 32687-78-8
- Min. Order:
- 20KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month