8-Hydroxyquinoline: Pharmacodynamics, Toxicity, Applications, Preparation
Introduction
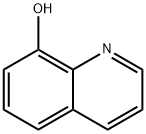
8-Hydroxyquinoline, also known as oxine or 8-quinolinol, is an organic compound with the molecular formula C9H7NO. It is a yellow solid that is soluble in organic solvents but has low solubility in water. 8-Hydroxyquinoline and its derivatives have a wide range of applications in various fields, including chemistry, medicine, and materials science.It is important to note that 8-hydroxyquinoline and its derivatives can be toxic if ingested or inhaled in large quantities. Therefore, appropriate safety measures and handling procedures should be followed when using these compounds[1].

Figure 1 appearance of 8-hydroxyquinoline
Pharmacodynamics
Pharmacodynamics of 8-Hydroxyquinoline refers to the study of the biochemical and physiological effects of this compound on living organisms. 8-Hydroxyquinoline is a synthetic organic compound that has been found to have a wide range of pharmacological activities, including antimicrobial, antiviral, and anticancer properties.
One of the primary mechanisms of action of 8-Hydroxyquinoline is its ability to chelate with metal ions such as zinc and copper, which are essential for the growth and survival of many pathogenic microorganisms. This process disrupts the normal functioning of these microorganisms, leading to their death or inhibition of growth.
In addition to its antimicrobial properties, 8-Hydroxyquinoline has also been shown to possess antitumor activity by inducing apoptosis (programmed cell death) in cancer cells. The compound has been investigated as a potential treatment for various types of cancer, including lung cancer, breast cancer, and colon cancer.
Overall, the pharmacodynamics of 8-Hydroxyquinoline suggest that it has broad therapeutic potential for a variety of infectious and neoplastic diseases, making it an important compound for continued research and development in the field of medicine[2].
Toxicity
8-Hydroxyquinoline is considered to be relatively low in toxicity for humans and other mammals. However, it may cause skin and eye irritation upon direct contact, and it can potentially cause respiratory irritation if inhaled in large quantities. Animal studies have shown that high doses of 8-hydroxyquinoline can cause liver and kidney damage, as well as adverse effects on the central nervous system. Prolonged exposure to the compound has also been linked to developmental and reproductive toxicity in animals. In addition, some studies have suggested that 8-hydroxyquinoline and its derivatives may have endocrine-disrupting effects, potentially interfering with hormonal pathways. It is important to handle 8-hydroxyquinoline with care and follow all relevant safety protocols and guidelines. Proper protective equipment should be worn, including gloves and safety glasses, when working with this substance. It is also important to avoid inhalation of the compound and to work in a well-ventilated area.
Overall, while 8-hydroxyquinoline is relatively low in toxicity, appropriate safety measures and handling procedures should be followed in order to minimize potential health risks[3].
Applications
In chemistry, 8-hydroxyquinoline is commonly used as a chelating agent to form complexes with metal ions such as aluminum, copper, and iron. This makes it useful in analytical chemistry and separation techniques. it is also used in the synthesis of other organic compounds, including dyes, drugs, and polymerization initiators. In medicine, its derivatives have been investigated for their potential therapeutic applications. They have been shown to possess anti-cancer, anti-viral, and anti-inflammatory properties, among others. One derivative, clioquinol, has been used as an antibiotic and anti-fungal agent. In materials science, its derivatives have been used in the fabrication of organic light-emitting diodes (OLEDs) and other electronic devices. Their ability to form stable complexes with metal ions makes them useful as dopants and charge transport materials[4].
Preparation
There are different methods for synthesizing 8-hydroxyquinoline. One of the most common methods is the Skraup synthesis, which involves the condensation of aniline with glycerol, sulfuric acid, and nitrobenzene, followed by oxidative cyclization with nitrobenzene.
Here is a simplified overview of the Skraup synthesis of 8-hydroxyquinoline:
Step 1: Condensation - glycerol, aniline, and sulfuric acid are mixed together and heated to form a reaction mixture. Nitrobenzene is then added dropwise while the mixture is stirred.
Step 2: Oxidative Cyclization - the reaction mixture is further heated to around 160-180℃, resulting in the formation of the intermediate 8-nitroquinoline. The nitro group is then reduced to an amino group by treatment with iron filings or another reducing agent, followed by oxidation with chromic acid to form 8-hydroxyquinoline.
Another route to synthesize 8-hydroxyquinoline is via the Borsche–Drechsel cyclization of o-nitrophenylaldehyde with formamide or its derivatives. Other methods that have been reported include the Pfitzinger reaction, the Bucherer reaction, and the Friedländer synthesis.
It is important to note that these methods require appropriate safety measures and handling procedures[5].
References
[1] Chiter Fatah,Costa Dominique,Pébère Nadine,Marcus Philippe,LacazeDufaure Corinne. Correction: Insight at the atomic scale of corrosion inhibition: DFT study of 8-hydroxyquinoline on oxidized aluminum surfaces.[J]. Physical chemistry chemical physics : PCCP,2023.
[2] Xu, X., Lv, X., Wang, Y., et al. Synthesis, structure, and antimicrobial activity of 8-hydroxyquinoline derivatives against gram-positive pathogens. [J] Journal of agricultural and food chemistry. 2020, 68(23), 6357–6366.
[3] Wang Y, Chen L, Chen C Y, et al. Potential enzyme-mediated linkages between hydroxyquinoline and nitrogen metabolism in the coastal microbial community[J]. Environmental Pollution, 2020, 267: 115465.
[4] Vaghefinazari Bahram,Lamaka Sviatlana V.,Blawert Carsten,Serdechnova Maria,Scharnagl Nico,Karlova Polina,Wieland D.C.Florian,Zheludkevich Mikhail L.. Exploring the corrosion inhibition mechanism of 8-hydroxyquinoline for a PEO-coated magnesium alloy[J]. Corrosion Science,2022,203.
[5] Ji S J, Wang X J, Cao Y, et al. A comprehensive review on the synthesis and applications of 8-hydroxyquinoline and its derivatives[J]. Journal of Materials Chemistry C, 2019, 7(33): 10381-10404.
);You may like
Related articles And Qustion
See also
Lastest Price from 8-Hydroxyquinoline manufacturers

US $1.00/g2024-04-27
- CAS:
- 148-24-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00/Kg/Drum2024-04-26
- CAS:
- 148-24-3
- Min. Order:
- 1KG
- Purity:
- ≥99.5%
- Supply Ability:
- 500mt