5-Chloro-2-nitroaniline: properties, applications and safety
General Description
5-Chloro-2-nitroaniline is a yellow crystalline solid with moderate solubility in organic solvents. It possesses strong electron-withdrawing groups, making it reactive in various chemical reactions. However, it is sensitive to light and heat and requires careful handling. Its applications lie in pharmaceutical research, where it serves as a key building block for the synthesis of sorafenib analogues with potential anticancer properties. These analogues have shown cytotoxic activity against HeLa and MCF-7 cancer cell lines. Safety precautions are crucial when dealing with 5-Chloro-2-nitroaniline as it is highly toxic to humans and the environment, posing risks to internal organs and aquatic life.

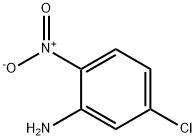
Figure 1. 5-Chloro-2-nitroaniline
Properties
5-Chloro-2-nitroaniline is an organic compound with the molecular formula C6H5ClN2O2. It belongs to the class of aromatic amines and is characterized by its unique properties. Firstly, 5-Chloro-2-nitroaniline is a yellow crystalline solid with a melting point of approximately 125-129°C. It exhibits moderate solubility in organic solvents such as ethyl acetate and dichloromethane, but is relatively insoluble in water. Secondly, this compound is notable for its strong electron-withdrawing groups, namely the chlorine and nitro substituents. These electron-withdrawing groups enhance its reactivity in various chemical reactions, making it a useful intermediate in pharmaceutical and dye synthesis. Additionally, 5-Chloro-2-nitroaniline is sensitive to light and heat. Prolonged exposure to light or elevated temperatures can result in degradation or decomposition of the compound, leading to changes in its properties. In summary, 5-Chloro-2-nitroaniline is a yellow crystalline solid with moderate solubility in organic solvents. It possesses strong electron-withdrawing groups, making it reactive in various chemical reactions. However, it is sensitive to light and heat and requires careful handling. 1
Applications
5-Chloro-2-nitroaniline is a chemical compound with diverse applications in pharmaceutical research. It serves as a key building block for the synthesis of novel sorafenib analogues containing a quinoxalinedione ring and amide linker. These analogues are potential anticancer agents that exhibit cytotoxic activity against HeLa and MCF-7 cancer cell lines. The synthesis of these compounds involves several synthetic steps. The synthesis begins by protecting the amino group of p-aminophenol. Subsequently, O-arylation occurs with 5-chloro-2-nitroaniline. The next step involves reducing the nitro group, followed by cyclization of the diamine group with oxalic acid. This cyclization reaction leads to the formation of a new compound. Evaluation of the synthesized compounds reveals their cytotoxicity against HeLa and MCF-7 cancer cell lines. Notably, some compounds exhibit significant cytotoxic activity, with IC50 values ranging from 16 to 25 μM against both cell lines. In summary, 5-Chloro-2-nitroaniline plays a crucial role in the synthesis of sorafenib analogues with potential anticancer properties, demonstrating cytotoxic activity against HeLa and MCF-7 cancer cell lines. Further research and development of these compounds may contribute to the advancement of pharmaceutical options for cancer treatment. 2
Safety
5-Chloro-2-nitroaniline is a highly toxic and hazardous chemical compound that poses significant risks to human health and the environment. The compound is fatal if swallowed, inhaled or in contact with skin. Even prolonged or repeated exposure to this substance may cause damage to internal organs. Therefore, it is important to handle and store this chemical with extreme care, using appropriate personal protective equipment and following all necessary safety measures. In addition, 5-Chloro-2-nitroaniline is toxic to aquatic life with long-lasting effects. If released into the environment, it can cause significant harm to aquatic ecosystems and biodiversity. Therefore, it is essential to prevent spills and carefully manage the disposal of the compound. Furthermore, 5-Chloro-2-nitroaniline falls under the category of self-reactive substances and mixtures - Type G, indicating that it may undergo a strongly exothermic decomposition even without participation of oxygen, leading to explosion hazards. To ensure the safe handling of 5-Chloro-2-nitroaniline, it is recommended to strictly follow all safety guidelines and precautions, including proper storage, labeling, and handling procedures, as well as appropriate training for individuals who may come into contact with the substance. 3
Reference
1. PubChem. COMPOUND SUMMARY: 5-Chloro-2-nitroaniline. National Library of Medicine, 2005, PubChem CID: 74218.
2. Sadeghian-Rizi S, Khodarahmi G, Sakhteman A, Jahanian-Najafabadi A, Rostami M, Mirzaei M, Hassanzadeh F. Synthesis and characterization of some novel diaryl urea derivatives bearing quinoxalindione moiety. Res Pharm Sci. 2018 Feb;13(1):82-92.
3. Summary of Classification and Labelling: 5-chloro-2-nitroaniline. European Chemicals Agency, EC/List no. 216-661-5.
);You may like
Related articles And Qustion
See also
Lastest Price from 5-Chloro-2-nitroaniline manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 1635-61-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00-0.00/kg2023-12-29
- CAS:
- 1635-61-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1 ton