4,4'-Methylenedianiline: properties, applications and body burden
General Description
4,4'-Methylenedianiline is a chemical compound with various physical and chemical properties, including a faint fishlike odor, insolubility in water, and solubility in alcohol, benzene, and ether. It has numerous applications in different industries, such as the production of polyurethane foams, epoxy resins, urethane elastomers, adhesives, and rubber processing chemicals. However, it is also a carcinogen and has restricted use due to potential health risks. Studies on workers exposed to 4,4'-Methylenedianiline indicated a decrease in its levels over time and provided insights into its elimination and accumulation in the body.

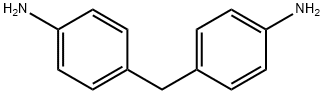
Figure 1. 4,4'-Methylenedianiline
Properties
4,4'-Methylenedianiline exhibits various chemical and physical properties. With a molecular weight of 198.26 g/mol, it appears as tan flakes or lumps and has a faint fishlike odor. It is insoluble in water and can be found in the form of dry powder, other solid, pellets, or large crystals. When crystallized from water or benzene, it forms tan flakes, lumps, or pearly leaflets. The compound has a faint, amine-like odor and a boiling point of 398 °C. Its melting point ranges from 91.5 to 92 °C, and it has a flash point of 220 °C. 4,4'-Methylenedianiline is slightly soluble in cold water but very soluble in alcohol, benzene, and ether. It has a density of 1.15 at 77 °F, making it denser than water, and a vapor density of 6.8, indicating that it is heavier than air. The compound oxidizes in air, turning from pale yellow to dark color. When heated to decomposition, it emits highly toxic fumes of aniline and NOx. Additionally, it is classified as a weak base with a logP value of 1.6, indicating its hydrophobic nature. 1
Applications
4,4'-Methylenedianiline has various applications in different industries. It is primarily used in the production of 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates, which are essential for manufacturing polyurethane foams. 4,4'-Methylenedianiline also acts as a curing agent for epoxy resins and urethane elastomers, making it valuable in adhesive and adhesive remover products, including glues, paints, inks, dental bonding agents, and microelectronic encapsulations. Additionally, 4,4'-Methylenedianiline serves as a corrosion preventative for iron and an antioxidant for lubricating oils. It is utilized as a rubber processing chemical and as an intermediate in the production of elastomeric fibers like Spandex. 4,4'-Methylenedianiline plays a role in the preparation of azo dyes and is involved in the determination of tungsten and sulfates. Furthermore, the isocyanates derived from 4,4'-Methylenedianiline are incorporated into semiflexible polyurethane foams used for automobile safety cushioning. It should be noted that 4,4'-Methylenedianiline is listed as a carcinogen and has restricted use due to its potential health risks. 2
Body burden
4,4'-Methylenedianiline is a chemical compound used in various industrial processes. Body burden refers to the amount of a substance present in an individual's body. Analysis of urine samples collected from workers involved in MDA production showed a decrease in MDA levels over time. In 1970, 14.9% of samples had MDA levels above 200 ppb, while in 1980, only 0.09% of samples had levels above 20 ppb. This indicates a significant reduction in 4,4'-Methylenedianiline exposure. Further studies on workers exposed to 4,4'-diaminodiphenylmethane, which is used as a curing agent containing 4,4'-Methylenedianiline, revealed important information about its elimination and accumulation. Male workers at a specific work site showed an elimination rate of 0 to 90 μmol/hr. The cumulated excretion amount ranged from 0.04 to 1.2 μmol per day on workdays and 0.005 to 0.51 μmol daily on weekends. Blood concentrations of 4,4'-diaminodiphenylmethane in these workers ranged from 10 to 60 nmol/L. Moreover, urine samples collected at the end of work shifts from workers exposed to 4,4'-diaminodiphenylmethane contained average concentrations of 236 μg/g creatinine (August 1988), 98 μg/g creatinine (October 1988), 50 μg/g creatinine (June 1989), 202 μg/g creatinine (May 1990), and 43 μg/g creatinine. These findings provide insights into the body burden and elimination of 4,4'-Methylenedianiline in workers exposed to it. 3
Reference
1. 4,4'-Methylenedianiline. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 7577.
2. PubChem Annotation Record for 4,4'-DIAMINODIPHENYLMETHANE. National Center for Biotechnology Information, 2023, Hazardous Substances Data Bank.
3. IARC; IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some Chemicals Used in Plastic and Elastomers. 1986, 39: 347-365.
You may like
Related articles And Qustion
See also
Lastest Price from 4,4'-Methylenedianiline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 101-77-9
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/KG2025-03-21
- CAS:
- 101-77-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month