3-Isopropylphenol: Harnessing Chemical Potentials While Navigating Toxicological and Safety Challenges
Introduction
3-Isopropylphenol, a compound known for its significant utility in the chemical synthesis and pharmaceutical industries, stands out due to its structural specificity and resultant biochemical activity. With a molecular backbone that enables it to interact uniquely with various substrates, this phenolic compound has garnered attention for its potential applications ranging from antimicrobial agents to intermediates in the synthesis of more complex chemical entities. Despite its valuable contributions, the use of 3-isopropylphenol raises important considerations regarding its environmental impact and safety profile, necessitating a closer examination by professionals in the field[1].

Figure 1 Characteristics of3-ISOPROPYLPHENOL
Mechanism of Action
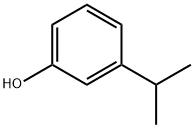
3-Isopropylphenol, a monohydric phenol derivative, is characterized by its isopropyl group attached to the phenol ring, a modification that significantly influences its chemical behavior and utility. The presence of the isopropyl group not only makes 3-isopropylphenol more lipophilic compared to its phenol precursors but also affects its electronic properties, thereby altering its reactivity and interaction with biological systems.
At the molecular level, 3-isopropylphenol participates in a variety of chemical reactions. It serves as a crucial precursor in the synthesis of complex organic compounds, including pharmaceuticals, where its phenolic hydroxyl group acts as a reactive site for etherification and esterification reactions. This hydroxyl group, being a potent nucleophile, facilitates the formation of bonds with electrophilic carbon atoms in organic syntheses, making 3-isopropylphenol a valuable building block for constructing more intricate molecular architectures.
Moreover, the biochemical efficacy of 3-isopropylphenol extends to its antimicrobial properties. The compound's mechanism of action against bacteria and fungi primarily involves the disruption of microbial cell membranes. Its lipophilic nature allows it to integrate into lipid bilayers, altering membrane fluidity and permeability, which ultimately leads to cell lysis and death. This mode of action, combined with its relative stability and solubility in organic solvents, makes 3-isopropylphenol a candidate for inclusion in antiseptic formulations and preservatives.
In industrial applications, the unique properties of 3-isopropylphenol are exploited in the production of polymers and resins. Its ability to undergo oxidative coupling reactions contributes to the formation of polymeric materials with desired mechanical and thermal properties. The controlled polymerization processes, facilitated by the phenolic structure of 3-isopropylphenol, result in high-performance materials suitable for a wide range of applications, from automotive components to consumer electronics.
Toxicity
The toxicological profile of 3-isopropylphenol is an essential aspect of its use in various industries, necessitating a thorough understanding of its impact on health and the environment. Research and regulatory studies have highlighted several key areas of concern regarding the exposure to and handling of this chemical compound.
Human exposure to 3-Isopropylphenol, primarily through inhalation, skin contact, or ingestion, can lead to adverse health effects. Acute exposure has been associated with irritation of the skin, eyes, and respiratory tract. Given its phenolic nature, 3-isopropylphenol can denature proteins and disrupt cell membranes, leading to cytotoxic effects. Furthermore, prolonged or repeated exposure may exacerbate these irritant effects, potentially leading to more severe dermatitis or respiratory issues[2].
In terms of environmental toxicity, 3-isopropylphenol poses risks to aquatic life. Its moderate solubility in water allows it to persist in aquatic environments, where it can exert toxic effects on fish and microorganisms. The compound's potential to bioaccumulate and disrupt aquatic ecosystems has been a subject of study, with research indicating that it can inhibit the growth and reproduction of certain species, thereby impacting biodiversity and the health of aquatic habitats.
The biodegradation of 3-isopropylphenol in soil and water systems is another factor to consider. While it is susceptible to microbial degradation, the rate and extent of this process can vary significantly depending on environmental conditions, potentially leading to the accumulation of the compound in certain settings. This variability underscores the importance of managing waste and emissions containing 3-isopropylphenol to mitigate its environmental footprint.
Given these toxicity concerns, regulatory bodies have established guidelines and exposure limits to ensure the safe handling and use of 3-isopropylphenol in industrial and laboratory settings. These regulations are designed to protect workers, consumers, and the environment from the potential hazards associated with this compound.
Precautions
Given the toxicity profile of 3-isopropylphenol, industries and laboratories must adopt stringent precautions and safety measures to mitigate risks associated with its handling, storage, and disposal. These guidelines are designed not only to protect workers and the environment but also to ensure compliance with regulatory standards.
Personal Protective Equipment (PPE)
The use of appropriate PPE is crucial when handling 3-isopropylphenol. This includes protective gloves, eyewear, and clothing resistant to chemicals to prevent skin and eye contact. In environments where inhalation risks exist, the use of respirators or appropriate ventilation systems is recommended to avoid respiratory exposure.
Storage
3-Isopropylphenol should be stored in a cool, well-ventilated area away from direct sunlight and sources of ignition. Containers must be tightly sealed and clearly labeled to prevent accidental exposure or mixing with incompatible substances. Since 3-isopropylphenol can interact with certain materials, using containers made of materials resistant to chemicals is necessary to prevent leaks or degradation.
Spill and Leak Procedures
In the event of a spill or leak, prompt action is required to contain and neutralize the substance. This involves using absorbent materials designed for chemical spills, ventilating the area, and disposing of waste according to local environmental regulations. Emergency procedures should be clearly outlined and accessible to all personnel involved in the handling of 3-Isopropylphenol.
Disposal
Disposal of 3-Isopropylphenol must adhere to environmental regulations and guidelines, emphasizing the minimization of its release into the environment. This includes contracting with certified waste disposal firms that can process chemical waste safely and efficiently, ensuring that it does not contribute to pollution or pose a risk to public health.
Training and Awareness
Regular training sessions and updates on safety protocols are essential for all personnel working with 3-isopropylphenol. Understanding the compound's properties, potential hazards, and emergency response measures is critical for maintaining a safe working environment.
References
[1]Choi K, Sweet L I, Meier P G, et al. Aquatic toxicity of four alkylphenols (3‐tert‐butylphenol, 2‐isopropylphenol, 3‐isopropylphenol, and 4‐isopropylphenol) and their binary mixtures to microbes, invertebrates, and fish[J]. Environmental Toxicology: An International Journal, 2004, 19(1): 45-50.
[2]Kr?mar S. Responses of Tabanidae (Diptera) to canopy traps baited with 4-methylphenol, 3-isopropylphenol, and naphthalene[J]. Journal of Vector Ecology, 2007, 32(2): 188-192.
You may like
Related articles And Qustion
Lastest Price from 3-ISOPROPYLPHENOL manufacturers

US $10.00/KG2022-06-02
- CAS:
- 618-45-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $10.00/Kg/Bag2022-06-02
- CAS:
- 618-45-1
- Min. Order:
- 1Kg/Bag
- Purity:
- 99%
- Supply Ability:
- 20 Tons