2,2',4,4'-Tetrahydroxybenzophenone: mechanism of action, pharmacokinetics and toxictiy
General Description
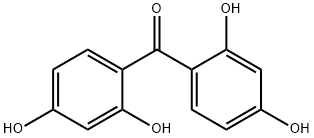
2,2',4,4'-Tetrahydroxybenzophenone is a sunscreen that affects melanin production. It weakly inhibits the tyrosinase enzyme involved in melanin synthesis and promotes the conversion of dopachrome to melanin. It hinders the hydroxylation process, preventing fully functional melanin formation. It cannot be hydroxylated itself. The inhibition is competitive. In terms of pharmacokinetics, it undergoes biotransformation in the body and is mainly metabolized by tissues other than the liver. The concentration of BP2 and its metabolites peak at 30 minutes after application and gradually decrease. BP2 is rapidly eliminated, while its metabolites are excreted in high amounts. However, 2,2',4,4'-Tetrahydroxybenzophenone can be toxic. Acute exposure can cause nausea, vomiting, and skin irritation, while chronic exposure may lead to endocrine disruption, DNA damage, and allergies. Its UV absorption and interaction with DNA generate reactive oxygen species that harm cells and tissues.

Figure 1. 2,2',4,4'-Tetrahydroxybenzophenone
Mechanism of action
2,2',4,4'-Tetrahydroxybenzophenone is a sunscreen that has a dual effect on the melanin biosynthesis pathway. It functions as a weak inhibitor of tyrosinase, an enzyme involved in melanin production, and it also promotes the conversion of dopachrome to melanin. By interacting with the active site of the enzyme, 2,2',4,4'-tetrahydroxybenzophenone hinders the hydroxylation process, preventing the formation of fully functional melanin. Interestingly, despite its structural similarity to substrates, it cannot undergo hydroxylation itself, confirming its role as an inhibitory compound rather than an alternative substrate. Additionally, it exhibits an accelerated interaction with dopachrome, leading to an enhanced melanin synthesis process. The inhibition caused by it is competitive, with an apparent constant inhibition value of 2.02 ± 0.09 mM. Although this value is higher than those reported in previous studies, 2,2',4,4'-tetrahydroxybenzophenone can still be utilized effectively as a sunscreen without significantly affecting the natural melanogenesis process. 1
Pharmacokinetics
The pharmacokinetic characteristics of 2,2',4,4'-tetrahydroxybenzophenone (BP2) can be summarized as follows. BP2 undergoes biotransformation in the body, as indicated by the presence of glucuronide- and sulfate-conjugates of BP2 in serum. BP2 does not undergo metabolization when tested with a S9 liver extract, suggesting that hepatic enzymes may not be responsible for its first-pass metabolism. Other tissues, such as the gut wall, might be involved in its initial metabolism after oral administration. The majority of absorbed BP2 is metabolized, as observed by comparing the concentrations of free BP2 and its metabolites in serum. The levels of both BP2 and its metabolites peak at 30 minutes after application and gradually decrease over time. The concentration of BP2-glucuronide is higher than that of BP2-sulfate, potentially due to limited availability of endogenous sulfur, which hampers the formation of sulfate conjugates compared to glucuronide conjugates. BP2 is rapidly eliminated from serum, with low amounts excreted directly, while the metabolites, particularly the glucuronide, are excreted in high amounts. The peak concentration of metabolites is observed at 120 minutes. Conjugation reactions, such as glucuronidation, play a crucial role in increasing solubility and promoting urine excretion of xenobiotics. 2
Toxicity
2,2',4,4'-Tetrahydroxybenzophenone is a chemical compound that is used as a UV filter in sunscreens, cosmetics, and personal care products. It absorbs UV radiation and acts as a shield against UV-induced damage to the skin. However, it has been associated with toxic effects in both acute and chronic exposures. Acute oral exposure to high doses can cause adverse effects such as nausea, vomiting, and diarrhea. Skin contact with high concentrations of the compound can result in irritation and allergic reactions. Inhalation may cause irritation of the respiratory tract, coughing, and shortness of breath. Chronic exposure to low levels may result in cumulative toxic effects. Prolonged use of sunscreens containing it has been associated with the disruption of the endocrine system and reproductive functions. It has also been shown to cause DNA damage and mutations in cells. Additionally, it has been linked to allergic reactions and skin sensitization in some individuals. The toxic effects are mainly due to its ability to absorb UV radiation and interact with DNA. It can generate reactive oxygen species that can cause oxidative damage to cells and tissues. Additionally, it can disrupt the function of hormones and enzymes involved in cellular metabolism and signaling. Overall, it is considered a toxic compound, especially in prolonged or excessive exposures. Usage of sunscreens containing 2,2',4,4'-tetrahydroxybenzophenone should be limited, and alternative sunscreen filters should be considered. 3, 4
Reference
1. Garcia-Jimenez A, Teruel-Puche JA, Garcia-Ruiz PA, Berna J, Rodríguez-López JN, Tudela J, Garcia-Canovas F. Action of 2,2',4,4'-tetrahydroxybenzophenone in the biosynthesis pathway of melanin. Int J Biol Macromol, 2017, 98:622-629.
2. Schlecht C, Klammer H, Frauendorf H, Wuttke W, Jarry H. Pharmacokinetics and metabolism of benzophenone 2 in the rat. Toxicology, 2008, 245(1-2):11-17.
3. Broniowska ?, Tomczyk I, Grzmil P, Bystrowska B, Skórkowska A, Maciejska A, Kazek G, Budziszewska B. Benzophenone-2 exerts reproductive toxicity in male rats. Reprod Toxicol, 2023, 120:108450.
4. Amar SK, Goyal S, Srivastav AK, Chopra D, Ray RS. Combined effect of Benzophenone-2 and ultraviolet radiation promote photogenotoxicity and photocytotoxicity in human keratinocytes. Regul Toxicol Pharmacol, 2018, 95:298-306.
);Related articles And Qustion
Lastest Price from 2,2',4,4'-Tetrahydroxybenzophenone manufacturers

US $0.00/KG2024-05-13
- CAS:
- 131-55-5
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 20 tons

US $5.00/kg2024-05-13
- CAS:
- 131-55-5
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 500mt/year