1,4-Butane Sultone: A Dual-Edged Sword in Green Chemistry and Fuel Cell Innovation
General Description
1,4-Butane sultone is a chemical compound of significant industrial and scientific interest due to its roles in both the synthesis of Brønsted acidic ionic liquids, aligning with green chemistry principles, and as a functionalizing agent in fuel cell technology. It enhances the mechanical properties and performance of fuel cells by contributing to the development of novel electrolytes with superior proton conductivity and power density when compared to traditional membranes like Nafion® 117. Despite its utility, 1,4-Butane sultone poses health risks, including potential carcinogenicity and environmental harm, necessitating careful handling, use of personal protective equipment, and adherence to safety regulations to mitigate exposure and environmental impact.

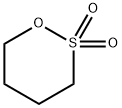
Figure 1. 1,4-Butane sultone
Properties
1,4-Butane sultone is a chemical compound with notable significance in both industrial and scientific contexts, particularly due to its role as an impurity in sulfobutyl ether β-cyclodextrin (SBE-β-CD) and its weak carcinogenic properties. This compound is pivotal in the synthesis of Brønsted acidic ionic liquids, which are celebrated for their alignment with green chemistry principles, alongside their remarkable water solubility, thermal stability, specificity, and catalytic resilience. These characteristics underscore the utility of 1,4-butane sultone in the production of symmetrical 3,3'-diaryloxyoctanone compounds, thereby cementing its importance in organic synthesis and catalysis. Chemically, 1,4-butane sultone is identified as a viscous, clear liquid that can appear colourless to yellowish/brown, typically lacking any distinct odour. Despite its utility, caution is advised in its handling due to its ability to form explosive mixtures with air when vaporized, presenting significant explosion hazards. Furthermore, its application as an alkylating agent highlights its chemical significance, notwithstanding the concerns regarding its weak carcinogenic potential. This dual nature—valuable in chemical synthesis yet hazardous—emphasizes the need for careful management and application in chemical processes. 1
Fuel cell applications
1,4-Butane sultone plays a pivotal role in the functionalization of single-walled carbon nanotubes (SWCNTs), which are incorporated into cross-linked composite electrolyte membranes for fuel cell applications. This process significantly enhances the mechanical properties and performance of the fuel cells. Specifically, 1,4-Butane sultone, when used in conjunction with sulfanilic acid, contributes to the development of a novel class of electrolytes with improved physico-chemical characteristics. These electrolytes are part of a broader strategy to synthesize cross-linked composite membranes by integrating functionalized SWCNTs into a terpolymer matrix composed of phenolphthalein, 4,4′-difluorobenzophenone, and sodium 5,5′-carbonyl bis(2-fluorobenzene-sulfonate), further cross-linked with bisphenol-A diglycidyl ether for enhanced durability. The introduction of SWCNTs functionalized with 1,4-Butane sultone into the membrane matrix results in electrolytes that exhibit superior proton conductivity and fuel cell performance, compared to the commercially available Nafion® 117 membrane. Specifically, membranes incorporating 12 mass% of these functionalized SWCNTs demonstrated proton conductivities of 0.131 and 0.126 S cm^−1 at 80 °C with 100% relative humidity, and achieved high power densities of 0.43 and 0.39 W cm^−2, respectively. These advancements underscore the potential of 1,4-Butane sultone functionalized SWCNTs in creating more efficient and durable fuel cell membranes, offering a promising alternative to current technologies and supporting the ongoing development of sustainable energy solutions. 2
Safety precautions and environmental considerations
1,4-Butane sultone is a chemical compound associated with various safety concerns that necessitate careful handling and usage. It is harmful if swallowed, capable of causing skin irritation, serious eye irritation, and may even cause respiratory irritation. Beyond these immediate health risks, 1,4-butane sultone is also suspected of causing genetic defects and cancer, highlighting its potential as a mutagen and equivocal tumorigen. Its impact on aquatic life is also notable, with harmful effects that are long-lasting, underscoring the need for responsible disposal and environmental management. Studies have provided mixed results regarding its carcinogenic and mutagenic potential. For instance, experiments involving female ICR/HA Swiss mice injected subcutaneously with 1,4-butane sultone over an extended period did not show significant tumorigenic effects. However, other tests, such as the IP host-mediated assay and studies on Saccharomyces cerevisiae, have indicated positive results for mutagenic and recombinogenic activities at certain concentrations. The Ames test further confirmed its mutagenicity in several strains of Salmonella typhimurium without metabolic activation. Given these findings, it is crucial for individuals working with or exposed to 1,4-butane sultone to employ appropriate safety measures, including the use of personal protective equipment (PPE), ensuring proper ventilation in work areas, and adhering to guidelines for handling chemicals with potential carcinogenic and mutagenic properties. The compound's hazardous nature makes it imperative for industries and laboratories to follow strict regulations to mitigate health risks to humans and prevent environmental contamination. 3
Reference
1. 1,4-Butane sultone. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 15411.
2. Munavalli, Balappa B. Sachin N. Hegde and Mahadevappa Y. Kariduraganavar. Synthesis of cross‐linked composite membranes by functionalization of single‐walled carbon nanotubes with 1,4‐butane sultone and sulfanilic acid for fuel cell. Journal of Applied Polymer Science. 2022.
3. 1(4)-Butanesultone. European Chemicals Agency. EC / List no. 216-647-9.
);You may like
Related articles And Qustion
Lastest Price from 1,4-Butane sultone manufacturers

US $50.00/kg2024-04-20
- CAS:
- 1633-83-6
- Min. Order:
- 1kg
- Purity:
- 99.10%
- Supply Ability:
- 5000kg

US $0.00/kg2024-04-12
- CAS:
- 1633-83-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton