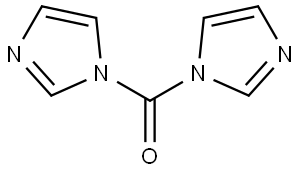
1,1'-Carbonyldiimidazole:Application and Preparation
1,1′-carbonyldiimidazole (CDI) is a white crystalline solid available in kilogram quantities. It i

Figure 1 Appearance of 1,1'-Carbonyldiimidazole.
CDI can also be used for esterification, although alcoholysis requires heat or the presence of a potent nucleophiles as sodium ethoxide, or other strong bases like NaH. This reaction has generally good yield and wide scope, although forming the ester from tertiary alcohols when the acid reagent has a relatively acidic α-proton is troublesome, since C-C condensations can occur, though this itself may be a desirable reaction [4]. Another related reaction is the reaction of formic acid with CDI to form the formylized imidazole. This reagent is a good formylating agent and can regenerate the unsubstituted imidazole (with formation of carbon monoxide) upon heating. Besides, CDI can act as a carbonyl equivalent in the formation of tetronic acids or pulvinones from hydroxyketones and diketones in basic conditions [5].
Preparation
CDI can be synthesized by single step according to the previous work [6]. 272.4 g of imidazole was added to 1400 g of chlorobenzene in a flask fitted with column and distillation bridge. 720 g of 30% strength methanolic sodium methoxide solution was added dropwise, and the methanol formed was removed by distillation. 300 ml of chlorobenzene was then removed by distillation, and 2 g of tributylhexadecylphosphonium bromide was added. 202 g of phosgene was introduced at 80-90°C over the course of one hour.
When the addition was complete, the mixture was stirred at 90°C for another 1 hour while a vigorous stream of nitrogen was passed through the flask. The precipitate then present was filtered at 80°C and rinsed with 200 g of chlorobenzene at 90°C. The filtrate and the washing liquid were combined and cooled to 0°C. The resultant precipitate was filtered, rinsed with 200 g of dry chlorobenzene and dried at 60°C in vacuum (250 ml). 234.5 g of carbonyldiimidazole having a purity of 97.4% was thus obtained in the form of colorless crystals. This corresponded to a yield of 72.3% of theory.
1H NMR (CDCl3): δ = 8.11 (s, 2H), 7.32 (s, 2H), 7.11 (s, 2H), 5.89 (s, 1H), 5.75 (s, 1H), 3.43 (q, 2H), 3.27 (d, 2H), 1.97 (m, 2H), 1.61-0.81 (m). 13C NMR (CDCl3): δ = 149.1, 135.7, 130.3, 115.9, 50.8, 39.2, 38.9, 38.4, 37.9, 36.1, 33.5, 33.4, 33.2, 30.6, 30.2, 29.7, 26.8, 26.6, 26.4, 26.1, 26.0, 25.9, 24.9, 10.9, 10.7, 10.6. FT-IR ν(cm-1) = 2960, 2922, 2853, 1734, 1542, 1522, 1462, 1379, 1285, 1244, 1215.
[2]Verma, S. K.; Ghorpade, R.; Pratap, A.; Kaushik, M. P. Solvent free, N,N′-carbonyldiimidazole (CDI) mediated amidation. Tetrahe- dron Lett. 2012, 53, 2373?2376.
[3]Armstrong; Wenju Li. N,N'-Carbonyldiimidazole. Encyclopedia of Reagents for Organic Synthesis. 2007, doi:10.1002/9780470842898.rc024.pub2.
[4]Staab. Syntheses Using Heterocyclic Amides (Azolides). Angewandte Chemie International Edition in English. 1962, 1 (7): 351–367.
[5]Jerris; et al. A Facile Synthesis of Simple Tetronic Acids And Pulvinones. Tetrahedron Letters. 1979, 47 (47): 4517–4520.
[6]Appel et al. Aggregation of Ureido-Pyrimidinone Supramolecular Thermoplastic Elastomers into Nanofibers: A Kinetic Analysis. Macromolecules, 2011, 44(17), 6776-6784.
);You may like
Related articles And Qustion
See also
Lastest Price from 1,1'-Carbonyldiimidazole manufacturers

US $9.00-70.00/g2024-04-28
- CAS:
- 530-62-1
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $5.00/kg2024-04-28
- CAS:
- 530-62-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200mt