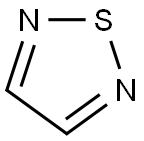
?Synthesis of 1,2,5-Thiadiazole
o adjacent nitrogen atoms (N-S-N). The pKa of –4.9 of the parent 1,2,5-thiadiazole indicates that it is a weaker base because of high aromaticity of the ring.
Synthesis
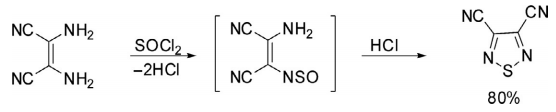
There are numerous protocols for the construction of 1,2,5-oxadiazoles but the most common approach is through the reaction of diamine with thionyl chloride. Diaminomaleonitrile on reaction with thionyl chloride afforded 1,2,5-thiadiazole-3,4-dicarbonitrile through the intermediacy of monosulfinylamine in 80% yields.
1. Parent 1,2,5-thiodiazole has been prepared from the reaction of ethylenediamine with sulfur monochloride, sulfur dichloride, or sulfur dioxide. Analogously, 3,4-dimethyl-1,2,5-thiadiazole has also been obtained from the reaction of 2,3-diaminobutane with SCl2.
2. Treatment of 2-aminoacetamides with thionyl chloride or sulfur monochloride in DMF at 22–90°C afforded 4-substituted 1,2,5-thiodiazoles in moderate to excellent yields.

Physical Properties
The parent 1,2,5-thiadiazole is a colorless, odorless liquid, with an mp of −50.1°C, a bp of 94°C, a density of 1.268 gm/mL–1, and is soluble in water, carbon tetrachloride, and chloroform; it is also thermally stable and has a high aromatic character. It is weakly basic with a pKa of −4.90 and a dipole moment of 1.56 D.
Chemical Reactivity
The high aromaticity of parent 1,2,5-thiadiazole gives high stability of the molecule. However, 3,4-diphenyl-1,2,5- thiadiazole is photodegradable producing benzonitrile with extrusion of sulfur. The high electron density in π-orbital compared to nitrogen loan pairs is the basis for the low basicity of the 1,2,5-thiadiazole ring. Because of low electron density on nitrogen, N-alkylation is not very facile, while ring sulfur oxidized readily with MCPBA to a nonaromatic thiadiazole-1-oxide and thiadiazole-2-oxide.
);
![132-65-0 Dibenzo[b,d]ThiopheneSynthesisPropertiesReactivityApplications](/NewsImg/2022-01-29/6377904812949559945959275.jpg)