(R)-DIFLUORPHOS(TM): Chemical synthesis and application

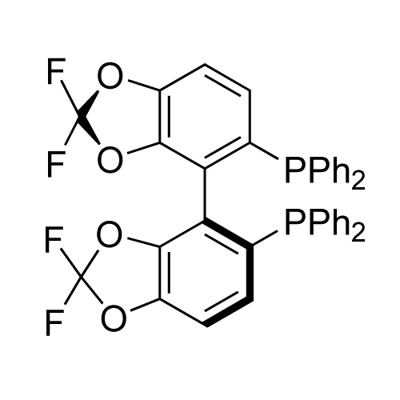
Figure 1 Appearance of (R)-DIFLUORPHOS(TM).
The (R)-DIFLUORPHOS(TM) can be synthesized according to the previous work [1]. The synthetic approach to (R)-DIFLUORPHOS(TM) (5; difluorphos) is depicted in Figure 2. Phosphorylation of commercially available 1 was through the organomagnesium formation, followed by the addition of chlorodiphenylphosphane oxide. Phosphane oxide 2 was obtained in 66% yield. Ortho-lithiation of 2 by LDA at À788C and further iodination with I 2 furnished 3 in 88% yield. Iodide 3 was subjected to an Ullmann-type coupling [2] with copper in DMF at 1308C and afforded the bis(phosphane oxide) 4 in 69% yield. The optical resolution of (RS)-4 was by chiral preparative HPLC using a Chirose C3 Column in 90% yield based on (RS)-4. (À)-4 and (+)-4 were enantiomerically pure (> 99% ee) according to HPLC (Chiralpak AD column). Reduction of the resolved 4 was performed by heating with an excess of HSiCl3 in xylene in the presence of tributylamine in 91% yields for (R)-5 and (S)-5 ((R)-difluorphos and (S)- difluorphos). (+)-(S)-difluorphos ((S)-5): m.p. > 2608C ; 1H NMR (300 MHz, CDCl3): d = 6.89 (dt, J = 1.5, 8.2 Hz, 2H), 7.02 (d, J = 8.2 Hz, 2H), 7.10–7.22 (m, 8H), 7.23–7.35 ppm (m, 12H); 31P NMR (162 MHz, CDCl3 , H3PO4 (85%)): d = À12.23 ppm; 19 F NMR (376 MHz, CDCl3 ): d = À49.90 ppm (dd, J = 160.8, 93.4 Hz); MS (EI): m/z: 683 [M+H]+ ; HR-MS: calculated for C38 H25 F4 O4 P2 [M+H] 683.1164, found 683.1147; [a] D 20 = + 20 (c = 0.1 in benzene). The reduction of (+)-(R)-4 afforded (À)-(R)-difluorphos ((R)-5) in 91% yield as a white solid. All analytical data were identical to the corresponding spectra of (+)-(S)-5. R enantiomer: [α] D 20 = À20 (c = 0.1 in benzene).
(R)-DIFLUORPHOS (TM) is used as a bidentate ligand in the synthesis of catalyst, which is specially designed for catalytic asymmetric hydrogenation. The advantage of asymmetric catalysis is that pure photoactive isomers can be directly prepared by asymmetric induction without the need to separate racemic mixtures. Furthermore, as a diphosphine ligand, (R)-DIFLUORPHOS (TM) is often used to prepare asymmetric catalytic metal complexes for the following reactions: hydrogenation, carbonylation, hydrosilylation, C-C bond formation and even the asymmetric isomerization of propylene amine [3]. (R)-DIFLUORPHOS (TM) is undoubtedly a powerful synthetic tool of the organic chemist on both a laboratory and a production scale. In recent years, progress in this field has been spectacular and ultimately recognized by the 2001 Nobel Prize awarded to Knowles [4] and Noyori [5] for enantioselective hydrogenation and to Sharpless [6] for enantioselective oxidation catalysis. In particular, enantioselective hydrogenation [7] using molecular hydrogen to reduce prochiral C=X (X=N,O) or C=C double bonds is one of the most efficient and versatile methods to build chiral compounds and valuable synthetic intermediates. Effective homogeneous hydrogenation catalysts are organometallic complexes that consist of one or more (chiral) ligands coordinated to an ionic metal center (Ru, Rh, Ir, . . .).
[2]J. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359.
[3]D. De Paule, C. Nicolas et al. Fr. Demande, 2830254, 04 Apr 2003.
[4]W. S. Knowles. Angew. Chem. Int. Ed. Engl. 2002, 41, 1998–2007.
[5]R. Noyori. Angew. Chem. Int. Ed. Engl. 2002, 41, 2008–2022.
[6]K. B. Sharpless. Angew. Chem. Int. Ed. Engl. 2002, 41, 2024–2032.
[7]R. Noyori. in Asymmetric Catalysis in Organic Synthesis (Wiley, New York), 1994, pp. 1–93.
);