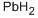Chemical Properties
| Melting point | 327.4 °C (lit.) |
| Boiling point | 1740 °C (lit.) |
| Density | 1.00 g/mL at 20 °C |
| refractive index | 2.881 (632.8 nm) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: soluble |
| form | wire |
| color | Olive-green or red to brown |
| Specific Gravity | 11.288 |
| Odor | Odorless gas |
| Resistivity | 20.65 μΩ-cm |
| Water Solubility | reacts with hot conc HNO3, boiling conc HCl, H2SO4 [MER06] |
| Merck | 13,5414 |
| Exposure limits | TLV-TWA 0.15 mg/m3 as Pb (ACGIH and MSHA), 0.05 mg (Pb)/m3 (OSHA); 10-h TWA 0.1 mg(inorganic lead)/m3 (NIOSH). |
| Stability | Stable. Incompatible with strong oxidizing agents, potassium, sodium. |
| CAS DataBase Reference | 7439-92-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 23, Sup 7) 1987 |
| EPA Substance Registry System | Lead (7439-92-1) |
Safety Information
| Hazard Codes | T,Xi,Xn,N |
| Risk Statements | 61-33-40-48/20-62-36/38-20/22-51/53-50/53-48/20/22-52/53-34-23/24/25 |
| Safety Statements | 53-45-61-36/37-36-26-60-36/37/39 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | OF7525000 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 78011000 |
| Hazardous Substances Data | 7439-92-1(Hazardous Substances Data) |
| Toxicity | LDLO oral (pigeon) 160 mg/kg PEL (OSHA) 0.05 mg/m3 PEL (action level) 0.03 mg/m3 TLV-TWA (ACGIH) 0.05 mg/m3 (PEL and TLV apply to lead and inorganic lead compounds) |
| IDLA | 100 mg Pb/m3 |
MSDS
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
Usage And Synthesis
Lead has numerous applications as metal, alloys and compounds. The major applications of the metal and its alloys such as solder are as materials of construction for pipe lines, plumbing fixtures, wires, ammunition, containers for corrosive acids and shield against short-wavelength radiation. Another major application is in storage batteries in which both the metal and its dioxide are used. Several lead compounds, such as lead chromate (chrome yellow), lead sulfate (white lead), lead tetroxide (red lead), and the basic carbonate are used in paints.

Lead has had a multitude of practical uses for over 8000 years and reports of poisoning exist in all ancient civilizations, including Greece, Rome, and China. By the second century in Greece, lead was known to cause colic when swallowed, and lead intoxication also produced paralysis.
2Pb + 2H2O + O2 → 2Pb(OH)2
In hard water, however, the presence of small amounts of carbonate, sulfate, or silicate ions form a protective film on the metal surface, and prevent the occurrence of the above reaction and thus, corrosion of the metal.
Lead does not evolve hydrogen readily with acids. Nitric acid attacks the metal readily, forming lead nitrate and oxides of nitrogen:
3Pb + 8HNO3 → 3Pb(NO3)2 + 2NO + 4H2O
This reaction is faster in dilute nitric acid than strong acid. Hydrochloric acid has little effect on the metal. At ordinary temperatures, lead dissolves slowly in hydrochloric acid, forming a coating of lead(II) chloride, PbCl2 over the metal, which prevents further attack.
At ordinary temperatures, lead is not readily attacked by sulfuric acid. A coating of insoluble lead sulfate formed on the metal surface prevents any further reaction of the metal with the acid. The acid is, therefore, stored in specially designed lead containers. Also, the action of hot concentrated sulfuric acid is very low up to about 200°C. However, at temperatures near 260°C, both the concentrated sulfuric and hydrochloric acids dissolve lead completely. At ordinary temperatures, hydrofluoric acid also has little action on the metal. Formation of insoluble PbF2 prevents dissolution of lead in the acid.
Organic acids in the presence of oxygen react slowly with lead, forming their soluble salts. Thus, acetic acid in the presence of oxygen forms lead(II) acetate:
2Pb + 4CH3COOH + O2 → 2Pb(CH3COO)2 + 2H2O
Lead dissolves in alkalies forming plumbite ion, Pb(OH)42¯ with the evolution of hydrogen:
Pb + 2OH¯ + 2H2O → Pb(OH)42¯ + H2
Lead combines with fluorine, chlorine, and bromine, forming bivalent lead halides:
Pb + Cl2 → PbCl2
Fusion with sulfur at elevated temperatures yields lead sulfide, PbS.
The metal is oxidized to PbO when heated with sodium nitrate at elevated temperatures.
Pb + NaNO3 → PbO + NaNO2
Lead is widely used in storage batteries. Each cell consists of a spongy lead plate as cathode and lead dioxide as anode immersed in the electrolyte sulfuric acid. The overall chemical reaction in the cell during discharge is as follows: PbO2 + Pb + 2H2SO4 → 2PbSO4 + 2H2O
The action level for lead in drinking water is 15μg/L. Its content in food and house paints is regulated in the USA by the Food and Drug Administration.
Lead is only slightly soluble in water. However, it is also toxic. This is the reason lead isno longer used to pipe fresh water into homes. It does not react well with acids, with theexception of nitric acid. Lead’s melting point is 327.46°C, its boiling point is 1,740°C, andits density is 11.342 g/cm3.
One of the most famous mining towns is the high-altitude western city of Leadville,Colorado. The boom started with the gold rush of the 1860s, followed by silver mining in the1870s and 1880s. Today, this city is the site of mining operations not only for lead, but alsofor zinc and molybdenum. At the height of its fame, Leadville had a population of almost50,000 people. Today the population is about 2,500.
Lead is commonly obtained by roasting galena (PbS) with carbon in an oxygen-rich environmentto convert sulfide ores to oxides and by then reducing the oxide to metallic lead.Sulfur dioxide gas is produced as a waste product. Large amounts of lead are also recoveredby recycling lead products, such as automobile lead-acid electric storage batteries. About onethirdof all lead used in the United States has been recycled.
Lead is obtained chiefly from galena (PbS) by a roasting process. Anglesite (PbSO4), cerussite (PbCO3), and minim (Pb3O4) are other common lead minerals. Lead is a bluish-white metal of bright luster, is very soft, highly malleable, ductile, and a poor conductor of electricity. It is very resistant to corrosion; lead pipes bearing the insignia of Roman emperors, used as drains from the baths, are still in service. Lead is used in containers for corrosive liquids (such as sulfuric acid) and may be toughened by the addition of a small percentage of antimony or other metals. Natural lead is a mixture of four stable isotopes: 204Pb (1.4%), 206Pb (24.1%), 207Pb (22.1%), and 208Pb (52.4%). Lead isotopes are the end products of each of the three series of naturally occurring radioactive elements: 206Pb for the uranium series, 207Pb for the actinium series, and 208Pb for the thorium series. Forty-three other isotopes of lead, all of which are radioactive, are recognized. Its alloys include solder, type metal, and various antifriction metals. Great quantities of lead, both as the metal and as the dioxide, are used in storage batteries. Lead is also used for cable covering, plumbing, and ammunition. The metal is very effective as a sound absorber, is used as a radiation shield around X-ray equipment and nuclear reactors, and is used to absorb vibration. Lead, alloyed with tin, is used in making organ pipes. White lead, the basic carbonate, sublimed white lead (PbSO4), chrome yellow (PbCrO4), red lead (Pb3O4), and other lead compounds are used extensively in paints, although in recent years the use of lead in paints has been drastically curtailed to eliminate or reduce health hazards. Lead oxide is used in producing fine “crystal glass” and “flint glass” of a high index of refraction for achromatic lenses. The nitrate and the acetate are soluble salts. Lead salts such as lead arsenate have been used as insecticides, but their use in recent years has been practically eliminated in favor of less harmful organic compounds. Care must be used in handling lead as it is a cumulative poison. Environmental concern with lead poisoning led to elimination of lead tetraethyl in gasoline. The U.S. Occupational Safety and Health Administration (OSHA) has recommended that industries limit airborne lead to 50 μg/cu. meter. Lead is priced at about 90¢/kg (99.9%).
When lead, which is very soft, is freshly cut, it has shiny blue-white sheen, which soonoxidizes into its familiar gray color. Lead is extremely malleable and ductile and can be workedinto a variety of shapes. It can be formed into sheets, pipes, buckshot, wires, and powder.Although lead is a poor conductor of electricity, its high density makes it an excellent shieldfor protection from radiation, including X-rays and gamma rays.
In the past, tetraethyl lead was added to gasoline to slow its burning rate in order to preventengine “knock” and increase performance. This caused serious and harmful pollution, and leadhas since been eliminated as a gasoline additive in most countries. Most exterior (and someinterior) house paints once contained high levels of lead as well. Today, the amount of lead inpaint is controlled, with not more than 0.05% allowed in the paint material.
Lead is used to make a number of important alloys. One is solder, an alloy of 1/2 lead and1/2 tin. Solder is a soft, low-melting metal that, when melted, is used to join two or moreother metals-particularly electrical components and pipes.
Babbitt metal is another alloy of lead that is used in the manufacture of wheel bearingsthat reduces friction. Lead is an ingredient in several types of glass, such as lead crystal andflint glass.
TV screens are coated with lead to absorb any radiation projected by the mechanism, andover 500,000 tons of lead is used in consumer electronics (computers, phones, games, and soon). Much of it ends up in solid waste dumps.
Many lead compounds are poisonous; thus, their uses in insecticides and house paints havebeen limited as other less toxic substances have been substituted. For example, lead arsenate[Pb3(AsO4], which is very poisonous, has been replaced in insecticides by less harmful substances.
All of the major soluble lead compounds have industrial uses. Lead acetate is used as a water repellent, for mildew protection, and as a mordant for cotton dyes. Lead acetate trihydrate is used in varnishes, chrome pigments, and as an analytical reagent, and lead chloride is used in asbestos clutch or brake linings, as a catalyst, and as a flame retardant. Lead nitrate is used in the manufacture of matches and explosives, as a heat stabilizer in nylon, and as a coating on paper for photothermography. Lead subacetate is used in sugar analysis and for clarifying solutions of organic substances (HSDB 2009).
The insoluble lead compounds also have a variety of uses. Lead azide and lead styphnate both are used in munitions manufacture. Lead carbonate, lead fluoride, lead fluoborate, and lead naphthenate are used as catalysts, with additional uses in the electronic and optical industries (lead fluoride), in coatings for thermographic copying (lead carbonate), as a curing agent for epoxy resins (lead fluoborate), and as a varnish drier (lead naphthenate). Lead phosphate and lead stearate both are used as stabilizers in the plastics industry. Lead iodide and lead sulfate are used in photography; lead iodide is also used in thermoelectric materials, and lead sulfate with zinc in galvanic batteries. Lead oxide and lead sulfide are used in ceramics; lead oxide is also used as a vulcanizing agent in rubber and plastics, and lead sulfide as a humidity sensor in rockets. Lead chromate is used as a pigment in paints, rubber, and plastics; lead tetraoxide is used in plasters, ointments, glazes, and varnishes; and lead thiocyanate is used in the manufacture of safety matches and cartridges. Lead arsenate formerly was used as an insecticide and herbicide, but no current uses were found.
Organic lead (including tetraethyl lead and tetramethyl lead) was widely used in the United States as an anti-knock additive in motorvehicle fuels until the U.S. Environmental Protection Agency initiated a phase-out of leaded gasoline in the early 1970s. By 1988, the total lead used in gasoline had been reduced to 1% of the 1970 level; in 1996, the use of lead in fuel for on-road motor vehicles was totally banned. Despite the legislated end to use of lead as a gasoline additive and reductions in some other uses of lead, overall U.S. lead consumption continued to grow until 1999, mainly because of increased production of lead-acid batteries (ATSDR 1999), but has since been on a general decline (USGS 2009, 2010, Guberman 2010).
Lead also occurs in various uranium and thorium minerals, arising directly from radioactive decay. Because certain isotopes are concentrated in lead derivatives from such sources, both the atomic weight and the density of the samples vary significantly from normal lead. Lead ores generally occur in nature in association with silver and zinc. Other metals commonly occurring with lead ores are copper, arsenic, antimony, and bismuth. Most of the world production of arsenic, antimony, and bismuth is a result of their separation from lead ores. Commercial lead ores may contain as little as 3% lead, but a lead content of 10% is most common. The ores are concentrated to ≥ 40% lead content before smelting. A variety of mechanical separation processes may be employed for the concentration of lead ores, but the sulfide ores are generally concentrated by flotation processes.
Workers in industries using lead are subject to testing of their blood and urine to determinethe levels of lead in their bodies’ organs. Great effort is made to keep the workers safe.
Unfortunately, many older homes (built prior to 1950) have several coats of lead-basedpaints that flake off, which then may be ingested by children, causing various degrees of leadpoisoning, including mental retardation or even death.
Young children are more susceptible to an accumulation of lead in their systems than areadults because of their smaller body size and more rapidly growing organs, such as the kidneys,nervous system, and blood-forming organs. Symptoms may include headaches, dizziness,insomnia, and stupor, leading to coma and eventually death.
Lead poisoning can also occur from drinking tap water contained in pipes that have beensoldered with lead-alloy solder. This risk can be reduced by running the tap water until it iscold, which assures a fresher supply of water.
Another hazardous source of lead is pottery that is coated with a lead glaze that is notstabilized. Acidic and hot liquids (citrus fruits, tea, and coffee) react with the lead, and eachuse adds a small amount of ingested lead that can be accumulative. Lead air pollution is stilla problem, but not as great as before, given that tetraethyl lead is no longer used in gasoline.However, lead air pollution remains a problem for those living near lead smelting operationsor in countries where leaded gasoline is still permitted.
Even though lead and many of its compounds are toxic and carcinogenic, our lives wouldbe much less satisfying without its use in our civilization.
Chronic exposure to inorganic lead via inhalation or ingestion can result in damage to the peripheral and central nervous system, anemia, and chronic kidney disease. Lead can accumulate in the soft tissues and bones, with the highest accumulation in the liver and kidneys, and elimination is slow. Lead has shown developmental and reproductive toxicity in both male and female animals and humans. Lead is listed by IARC in Group 2B ("possible human carcinogen") and by NTP as "reasonably anticipated to be a carcinogen," but is not considered to be a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
Acute toxic symptoms include ataxia,repeated vomiting, headache, stupor, hallucinations,tremors, convulsions, and coma.Such symptoms are manifested by the encephalopathicsyndrome. Chronic exposure can effects, anemia, and damage to the kidney.Lead can severely affect the nervous system.Chronic lead poisoning adversely affectsthe central and peripheral nervous systems,causing restlessness, irritability, and memoryloss. At lead concentrations of >80μg/dL,encephalopathy can occur. Cerebral edemaneuronal degenerationa and glial proliferationcan occur. The clinical symptoms areataxia, stupor, convulsion, and coma. Epidemiologicstudies in recent years have primarilyfocussed on the neurotoxic effectsof lead on children, especially in terms ofimpaired brain ability and behavioral problems.Permanent brain damage has beennoted among children from lead poisoning.Kidney damage arising from shorttermingestion of lead is reversible: whilea longer-term effect may develop to generaldegradation of the kidney, causing glomularatrophy, interstitial fibrosis, and sclerosisof vessels (Manahan 1989). Inhalation oflead justs can cause gastritis and changes inthe liver. Lead is significantly bioaccumulatedin bones and teeth, where it is storedand released. It binds to a number of cellularligands, interfering with some calciumregulatedfunctions. Lead has an affinity forsulfhydryl groups (-SH), which are presentin many enzymes. Thus it inhibits enzymaticactivity. One such effect is the inhibitionof δ-amino-levulinic acid dehydrates(ALAD) an enzyme required for the biosynthesisof heme, an iron(II)–porphyrin complexin hemoglobin and cytochrome. Anotherenzyme which is also highly susceptible tothe inhibitory effect of lead is heme synthetase.The impaired heme synthesis maycause anemia. The clinical anemia is perceptibleat a blood-lead level of 50 μg/dL. Concentrationsof lead in the blood at levels of10 μg/dL can cause ALAD inhibition. Carcinogenicityof lead has not been observedin humans; the evidence in animals is inadequate.
Suwalsky et al. (2003) studied the effectsof lead on the human erythrocyte membranes using isolated unsealed membranes andmolecular models consisting of bilayers ofdimyristoylphosphatidylcholine and dimyristoylphosphatidylethanolaminerepresentingphospholipids in the outer and inner monolayersof human erithrocyte membrane. Resultsof this study indicated that lead particlesadhered to the external and internal surfacesof human erithrocyte membrane and lead ionsinduced considerable molecular disorder inboth lipid multilayers.
Cremin et al. (1999) investigated the efficacyof chelation of lead with meso-2,3-dimercaptosuccinic acid in reducing the leadlevels in the brain and its neurotoxicity fromchronic oral exposure of the metal in adultrhesus monkeys. Their data, however, indicatedthat under the conditions of their studysuccimer treatment did not reduce brain leadlevels in the primate model and also the limitationsin the use of blood-lead level as anindicator of treatment efficacy.
With its high internal damping characteristics, lead is one of the most efficient sound attenuators for industrial, commercial, and residential applications. Sheet lead, lead-loaded vinyls, lead composites, and lead-containing laminates are used to reduce machinery noise. Lead sheet with asbestos or rubber sandwich pads are commonly used in vibration control.
Lead is obtained from its sulfide (PbS, galena), which is first roasted in the presence of oxygen and then reduced with carbon to give elemental Pb.
Lead is a greymetal and most lead is used in batteries.Other major uses, such as in plumbing or as antiknock agent in petrol (tetraethyl lead, Pb(C2H5)4), have declined over recent years because of the high toxicity of lead. Pb is a neurotoxin when ingested and many lead compounds are water soluble. Therefore, water lines have been replaced by specialised plastic material, and in most industrialised countries only unleaded petrol is sold.
The release of lead to air is now less than the release of lead to soil. Most of the lead in inner city soils comes from landfills and leaded paint. Landfills contain waste from lead ore mining, ammunition manufacturing, and other industrial activities such as battery production. Very little lead goes directly into water. Higher levels of lead from car exhausts can be measured near roadways. Very low levels of lead from car exhausts are found at distances of 25m (82 ft) from the road edge. However, once lead goes into the atmosphere, it may travel thousands of miles if the lead particles are small or if the lead compounds are volatile. Lead is removed from the air by rain as well as by particles falling to the ground or into surface water. Once lead deposits on soil, it usually sticks to soil particles. Small amounts of lead may enter rivers, lakes, and streams when soil particles are displaced by rainwater. Lead may remain stuck to soil particles in water for many years. Movement of lead from soil particles into underground water or drinking water is unlikely unless the water is acidic or ‘soft.’ Some of the chemicals that contain lead are broken down by sunlight, air, and water to other forms of lead. Lead compounds in water may combine with different chemicals depending on the acidity and temperature of the water. The lead atom cannot be broken down.
The levels of lead may build up in plants and animals from areas in which air, water, or soil are contaminated with lead. If animals eat contaminated plants or animals, most of the lead they eat will pass through their bodies. The small amount absorbed can cause harmful effects. The amount of lead in paints sold for consumer use may not exceed 0.06%. Releases from lead-based paints are frequently confined to the area in the immediate vicinity of painted surfaces, and deterioration or removal of the paint can result in high localized concentrations of lead in indoor air and on exposed surfaces. Sandblasting procedures to remove paint may disperse lead into the local environment.
The largest volume of organolead vapors released to the atmosphere results from industrial processes such as primary and secondary nonferrous metal smelting, and from the use of leaded gasoline, which contains tetraethyl lead as an antiknock additive. These vapors are photoreactive, and their presence in the local atmosphere is transitory. Halogenated lead compounds are also formed and, ultimately, oxides and carbonates. Tetra-alkyl lead compounds have been found to contribute 5–10% of the total particulate lead present in the atmosphere. Organolead vapors are most likely to occur in occupational settings (e.g., gasoline transport and handling operations, gas stations, and parking garages) and high traffic areas.
Although aquatic releases from industrial facilities are expected to be small, lead may be present in significant levels in drinking water. In areas receiving acid rain (e.g., northeastern United States) the acidity of drinking water may increase, thus increasing the corrosivity of the water, which may, in turn, result in the leaching of lead from water systems, particularly from older systems during the first flush of water through the pipes. Fish in more acidic waters accumulate more lead than fish in a more alkaline environment.
The grounding of household electrical systems to the plumbing can increase corrosion rates and the subsequent leaching of lead from the lead solder used for copper pipes. Areas in which the pH of the water is <8.0 may have higher lead drinking water levels as well.
Canning foods in lead-soldered cans may increase levels of lead 8- to 10-fold; however, the impact of canning appears to be decreasing as a result of a decrease in the use of leadsoldered cans. Additional exposure to lead through dietary intake by people living in an urban environment is estimated to be ~ 28 mg day-1 for adults and 91mg day-1 for children, all of which can be attributed to atmospheric lead (dust). Atmospheric lead may be added to food crops in the field or garden (through uptake from soil and from direct deposition onto crop surfaces), during transport to market, processing, and kitchen preparation.
Lead may leach from lead crystal decanters and glasses into the liquids they contain. Flaking paint, paint chips, and weathered powdered paint, which are most commonly associated with deteriorated housing stock in urban areas, are major sources of lead exposure for young children residing in these houses, particularly for children with pica (i.e., the compulsive, habitual consumption of nonfood items). Lead concentrations of 1000–5000 mg cm-2 have been found in chips of lead-based paint, suggesting that consumption of a single chip of paint would provide greater short-term exposure than any other source of lead.
Lead binds certain active groups on protein (e.g., sulfhydryl groups) and therefore may change the structure and function of certain proteins and enzymes. Lead interferes with the biosynthesis of heme in at least two steps in the multi-step process. Heme proteins are important to the structure and function of hemoglobin in red blood cells. Lead binds with 8-aminolevulinic acid dehydratase and depresses its activity. This biochemical block explains the occurrence of anemia found in chronic lead poisoning. Measurement of the blood levels of this enzyme is used as a test for lead intoxication. Lead also interferes with the incorporation of ferrous iron into the porphyrin ring. If iron is not attached to heme, then zinc will occupy the iron-binding site. The concentration of zinc protoporphyrin also can be used as a diagnostic tool for lead poisoning.
Preparation Products And Raw materials
- Lead monoxideTartrazineLead(II) nitrateLead acetate trihydrateMiddle chrome yellowCI NO 77605Lead chromatePale chrome yellowLEAD STEARATEN-Phenylanthranilic acidLead tetraacetatestarting type lead-acid storage batteryDark chrome yellowlead oxideLead silicateArsenic-lead alloyconductor fixing adhesive J-35
Lead manufacturers
Related articles
Lead-Hazard and ToxicitySep 9,2019
Related Product Information
The What'sApp is temporarily not supported in mainland China