Myelofibrosis (MF) is a rarely-occurring disease of myelodysplastic disorders. It is caused by the replacement of in vivo bone marrow by scar tissue, leading to the production of blood cells in the liver and spleen and other organs, which is characterized by splenomegaly, anemia, leukopenia, and thrombocytopenia, and different degrees of bone sclerosis. Symptoms include fatigue, abdominal discomfort, pain under the ribs, muscle and bone pain, itching and night sweats.
Ruxolitinib is the first oral medication approved by the US Food and Drug Administration (FDA) for the treatment of myelofibrosis. It is a small-molecule inhibitor of the tyrosine kinase (namely, JAK1 and JAK2) and is suitable for the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia Vera myelofibrosis and post essential thrombocythemia myelofibrosis.
August 29, 2012, the EU has approved the first drug for the treatment of myelofibrosis, ruxolitinib. Ruxolitinib can be used for the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia Vera myelofibrosis and post essential thrombocythemia myelofibrosis. Currently, ruxolitinib has been approved by more than 50 countries around the world, including the EU, Canada and some Asian, Latin American and South American countries.
US Novartis has obtained authorization from Incyte Company of the development and commercialization right of Ruxolitinib outside the United States. The European Commission and the FDA have both granted the status of Ruxolitinib as the orphan drug in the treatment of myelofibrosis. Currently, Incyte has sold it under the trade name Jakafi in the United States for the treatment of intermediate or high-risk myelofibrosis.

Figure 1 is the ruxolitinib tablet produced by the US Novartis Company (trade name: Jakafi)
Ruxolitinib, INCB18424 is a potent and selective JAK1 and JAK2 inhibitor (IC50 were 2.7 and 4.5nM, respectively). It has potent anti-tumor and immunomodulatory activity. Ruxolitinib can inhibit IL-6 signal (IC50 = 281nM) and the proliferation of JAK2V617F + Ba/F3 cell. Ruxolitinib can also inhibit the STA3 phosphorylation of wild-type JAK2 and JAK2 V617F mutant inside the body of patients. Clinical application of it can significantly cause reduction in the circulating levels of inflammatory cytokines. Ruxolitinib also has an effective role in model of the adjuvant arthritis and the multiple-cause murine arthritis.
This information is edited by Xiongfeng Dai from Chemicalbook.
Ruxolitinib is a kind of kinase inhibitor which is suitable for the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia Vera myelofibrosis and post essential thrombocythemia myelofibrosis.
1. For patients with platelet counts being greater than 200/μl, ruxolitinib should be applied at a start dose of 20 mg with being administered orally twice per day while for patients with platelet counts between 100/μl and 200/μl, they should apply a dose of 15mg twice a day.
2. Before starting to apply ruxolitinib treatment, you should perform complete blood count. Monitor the complete blood count in every 2-4 weeks and lower the adjusted dose for platelet count.
3. According to the result of the reaction, the patients can increase the dose to the maximum recommended dose of 25 mg twice per day. The patient should discontinue if the spleen gets no reduction or the symptoms are not improved, the patients should discontinue after 6 months.
The most common hematological adverse reactions (incidence> 20%) is lower platelet counts and anemia. The most common non-hematologic adverse reactions (incidence> 10%) are bruising, dizziness and headache.
Columbia University Medical Center has conducted a test of a drug designed to treat bone marrow diseases to investigate whether it can help people get rid of alopecia areata. The study found that, for human participating in human body test, including seven women and five men, after they have administrated a drug called Ruxolitinib, the symptoms of most people has been greatly alleviated. Part of their research has been published in the "Nature Medicine" magazine.
In November 2011, the U.S. FDA approved ruxolitinib (INCB018424)
for the treatment of patients with intermediate or high-risk myelofibrosis.
Ruxolitinib is
an ATP-competitive inhibitor of JAK1 and JAK2 (IC50's of 3.3±1.2 nM
and 2.8±1.2 nM, respectively) and inhibition occurs regardless of the
JAK2V617F mutational status. Ruxolitinib is a moderately potent inhibitor
of the related JAK, TYK2 (IC50=19±3.2 nM) but is selective versus
JAK3 (IC50=428±243 nM). It was also selective versus a panel of 26 other
kinases at concentrations approximately 100-fold the IC50 of JAK1 and
JAK2. Inhibition of JAK1 and JAK2 downregulates the JAK-signal transducer
and activator of transcription (STAT) pathway, inhibiting
myeloproliferation, inducing apoptosis, and reducing numerous cytokine
plasma levels.
Incyte Corporation (United States)
INCB018424 is the first potent, selective, JAK1/2 inhibitor to enter the clinic with IC50 of 3.3 nM/2.8 nM, >130-fold selectivity for JAK1/2 versus JAK3. Phase 3
A JAK family kinase inhibitor of JAK1, JAK2 and JAK3 with IC50s of 2.7 nM, 4.5 nM and 322 nM, respectively
Janus-associated kinases (JAKs) are cytoplasmic tyrosine kinases that are required for activating the signaling of certain cytokines and growth factor receptors. A JAK2 gene fusion mutation, JAK2V617F, that causes unchecked activation of various growth factors and cytokines, has been linked to myeloproliferative neoplasms (MPNs), including polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Ruxolitinib is a potent ATP mimetic that inhibits both JAK1 and JAK2 with IC50 values of 2.7 and 4.5 nM, respectively and is relatively less selective for JAK3 (IC50 = 322 nM). It can block interleukin-6 (IL-6) signaling (IC50 = 281 nM) and proliferation of JAK2V617F+ Ba/F3 cells (IC50 = 127 nM). In primary cultures, ruxolitinib preferentially suppresses erythroid progenitor colony formation from JAK2V617F+ polycythemia vera patients (IC50 = 67 nM) versus healthy donors (IC50 > 400 nM). In a mouse model of JAK2V617F+ MPN, 90 mg/kg ruxolitinib reduced splenomegaly, decreased circulating levels of IL-6 and TNF-α, eliminated neoplastic cells, and prolonged survival of the treated animals.[Cayman Chemical]
Ruxolitinib is a selective Janus tyrosine kinase (JAK1 and JAK2) inhibitor used in the treatment of myeloproliferative neoplasms and psoriasis.
The JAK family includes four isoforms, JAK1, JAK2, JAK3, and tyrosine kinase (TYK2). Ruxolitinib (Jakafi(R), Incyte Corp.) was the first approved JAK inhibitor, which inhibits both JAK1 and JAK2, used for the treatment of different types of myelofibrosis. Tofacitinib (Xeljanz(R), Pfizer) was approved by FDA as a JAK3-selective inhibitor for the treatment of rheumatoid arthritis and is one of the only two FDA-approved kinase inhibitors for non-oncological indications.
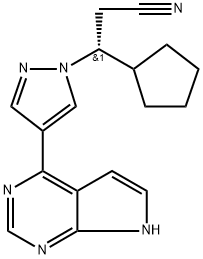
ChEBI: A pyrazole substituted at position 1 by a 2-cyano-1-cyclopentylethyl group and at position 3 by a pyrrolo[2,3-d]pyrimidin-4-yl group. Used as the phosphate salt for the treatment of patients with intermediate or high-risk myelofibrosis, includ
ng primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis.
Tyrosine kinase inhibitor:
Treatment of disease related splenomegaly or
symptoms in patients with primary myelofibrosis
(MF), post polycythaemia vera (PV) myelofibrosis
or post-essential thrombocythemia myelofibrosis
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
clarithromycin and telithromycin, reduced dose of
ruxolitinib; concentration reduced by rifampicin.
Antifungals: reduce dose of ruxolitinib with
fluconazole, itraconazole, ketoconazole, posaconazole
and voriconazole.
Antipsychotics: avoid with clozapine, risk of
agranulocytosis.
Antivirals: reduce dose of ruxolitinib with
boceprevir, indinavir, lopinavir, ritonavir, saquinavir
and telaprevir.
Ruxolitinib is mainly metabolised by CYP3A4 (>50%),
with additional contribution from CYP2C9 to produce
2 major and active metabolites. About 74% of a dose is
excreted in the urine and about 22% via the faeces.
1) Verstovsek?et al. (2009),?Therapeutic potential of JAK2 inhibitors; Hematology Am. Soc. Hematol. Educ. Program,?2009(1)?636
2) Quintas-Cardama?et al. (2010),?Preclinical characterization of the selective JAK1/2 inhibitor INCB01824: Therapeutic implications for the treatment of myeloproliferative neoplasms; Blood,?115?3109
3) Farr et al. (2017) Targeting cellular senescence prevents age-related bone loss in mice; Nat. Med. 23 1072
4) Li?et al.?(2017)?Identification of a novel functional JAK1 S646P mutation in acute lymphoblastic leukemia; Oncotarget?8?34687