Lignans
Lignans are natural organic compounds by a class of 2 or 3 molecules of benzene in different forms of polymerization, mainly located in the angiosperms and gymnosperms. It can be divided into two categories: The lignan is composed of two molecules of benzene and its derivatives through its side chain polymerization of β-bit. The new lignan is a molecular propyl benzene side chain, connected with benzene ring of another molecule, or the two part of the oxygen atom connected compound. The sesquilignan is a compound of three molecules of phenyl group. A compound condensed by the flavone or chalcone wiht allyl benzene derivatives, called flavonolignan or xanthonolignan. After lignan condensed by two molecule of phenylpropyl β-carbon, oxygen-containing groups of γ- carbon on the side chain can be substituted tetrahydrofuran / Hemiacetal / Lactone tetralin / cyclooctene structure formed by dehydration condensation. So lignans can be divided into more types. Such as monoe-poxy lignan, lignanolide, cycloli-gnan, etc. From the plant kingdom has been allocated more than 200 lignans, more than 100 new lignans. Chinese medicine Ciwujia, Schisandra, the brother Wang, burdock, Polygala, forsythia, Trachelospermum jasminoides, safflower, asarum, contain lignans.
In plants, lignans in the form of free or glucoside, most are optically active, almost all, and are mostly colorless crystals in free form, some of the new lignans as an oil. Lignans are soluble in organic solvents, sparingly soluble or insoluble in water. The solubility of glycosides in alcohols or water is larger, and can be hydrolyzed by acid. Extract lignans aforetime first proposed total lignans with methanol or ethanol, and then separate the single component by chromatography or fractional crystallization.
Most lignans components can be color in case of concentrated sulfuric acid. If the molecule of lignans contains a number of phenolic hydroxyl group, Such as ferric chloride reagents, alkaline reagents diazotized sulfanilic, can able to react with the phenolic reagent showed different colors. Some of the lignans can react with the five antimony chloride solution and carbon tetrachloride, such as, with podophyllotoxin reaction is brown, with alpha - peltatin is purple.
Many lignans component may have stereoisomers due to the saturated cyclic moiety. It has been found in some lignans unstable in traditional Chinese medicine, and it is easy to isomerization, changed into its solid isomer, influenced by acid or alkali. For example podophylloxin has significant anti-cancer effect, which is easily transformed into Picrop-odophyllin in alkaline solution; if affected by phosphorus trichloride, C1-OH translocation generated Epi-podophyllotoxin, and picrop-odophyllin and its derivatives had no anti-cancer activity.
We have found that lignans have a variety of activity, such as anti-cancer, anti-fungal, insecticidal, l white blood cells, lower alanine aminotransferase, liver, cough, diarrhea, etc. However, it is not used as a medicine. Derivatives of podophyllotoxin lignans useful as anti-cancer agents, lignans in Schisandra chinensis is known as lowering alanineaminotransferase , for the treatment of hepatitis.
- Structure:
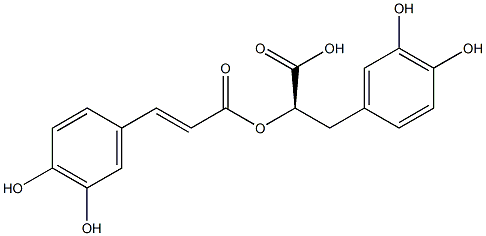
- Chemical Name:Rosmarinic acid
- CAS:20283-92-5
- MF:C18H16O8
- Structure:
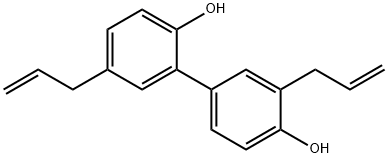
- Chemical Name:Honokiol
- CAS:35354-74-6
- MF:C18H18O2
- Structure:
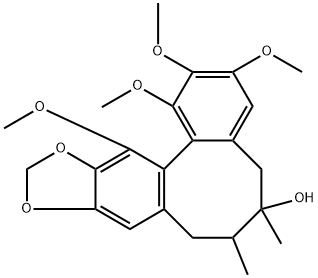
- Chemical Name:Gomisin A
- CAS:58546-54-6
- MF:C23H28O7
- Structure:
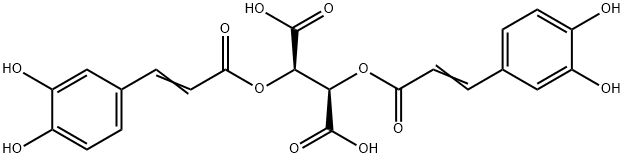
- Chemical Name:ChicoricAcid
- CAS:6537-80-0
- MF:C22H18O12
- Structure:
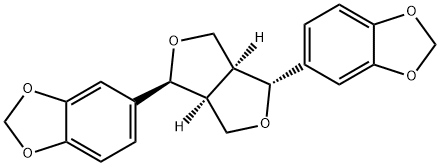
- Chemical Name:ASARININ
- CAS:133-04-0
- MF:C20H18O6
- Structure:
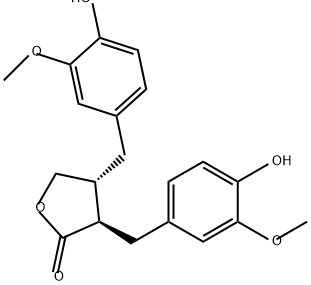
- Chemical Name:MATAIRESINOL
- CAS:580-72-3
- MF:C20H22O6
- Structure:
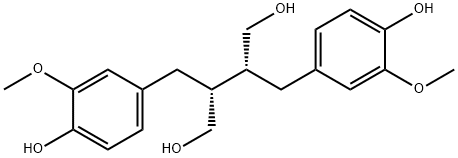
- Chemical Name:SECOISOLARICIRESINOL
- CAS:29388-59-8
- MF:C20H26O6
- Structure:
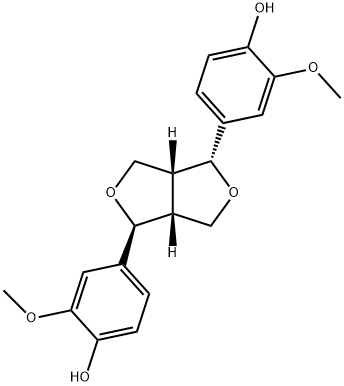
- Chemical Name:(+)-Epipinoresinol
- CAS:24404-50-0
- MF:C20H22O6
- Structure:
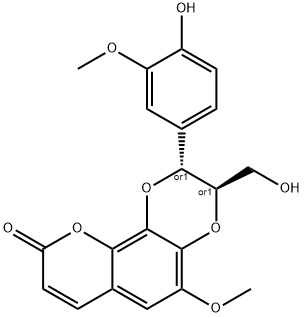
- Chemical Name:Cleomiscosin B
- CAS:76985-93-8
- MF:C20H18O8
- Structure:
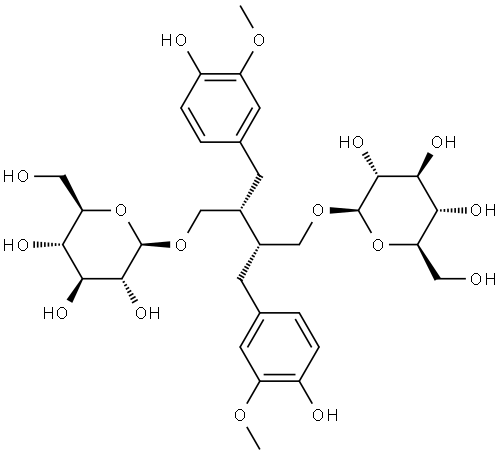
- Chemical Name:SECO-ISOLARICIRESINOL DIGLUCOSIDE
- CAS:158932-33-3
- MF:C32H46O16
- Structure:
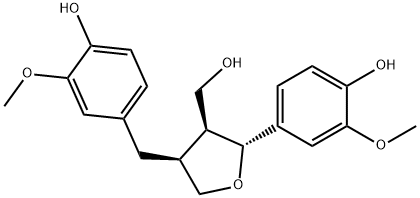
- Chemical Name:Tetrahydro-2-(4-hydroxy-3-methoxyphenyl)-4-((4-hydroxy-3-methoxyphenyl)methyl)-3-furanemethanol
- CAS:83327-19-9
- MF:C20H24O6
- Structure:
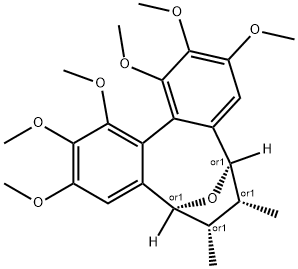
- Chemical Name:Kadsulignan N
- CAS:163564-58-7
- MF:C24H30O7
- Structure:
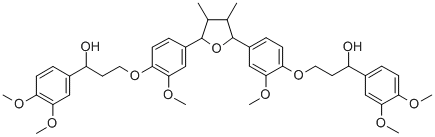
- Chemical Name:manassantin A
- CAS:88497-87-4
- MF:C42H52O11
- Structure:
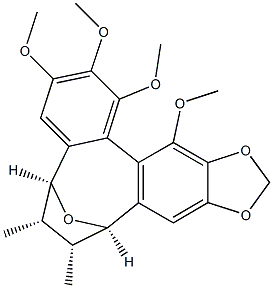
- Chemical Name:Kadsulignan L
- CAS:163660-06-8
- MF:C23H26O7
- Structure:
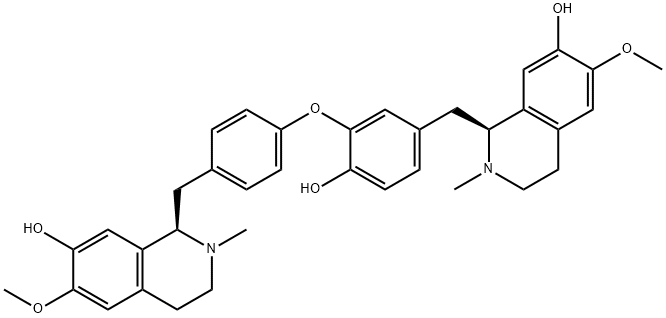
- Chemical Name:MAGNOLINE
- CAS:6859-66-1
- MF:C36H40N2O6
- Structure:
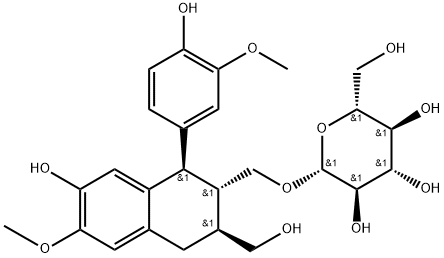
- Chemical Name:(-)-Isolariciresinol 9'-O-glucoside
- CAS:143236-04-8
- MF:C26H34O11
- Structure:
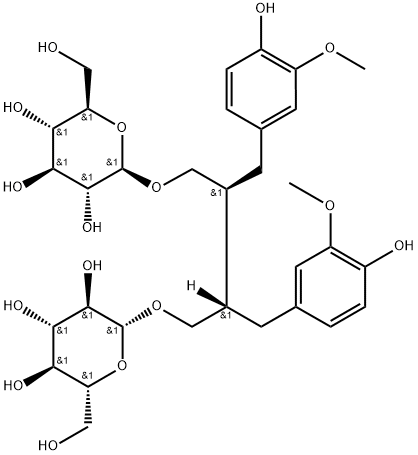
- Chemical Name:Secoisolariciresinol diglucoside
- CAS:257930-74-8
- MF:C32H46O16
- Structure:
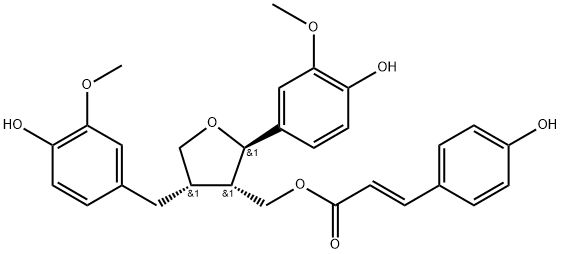
- Chemical Name:Lariciresinol p-coumarate
- CAS:864452-88-0
- MF:C29H30O8
- Structure:
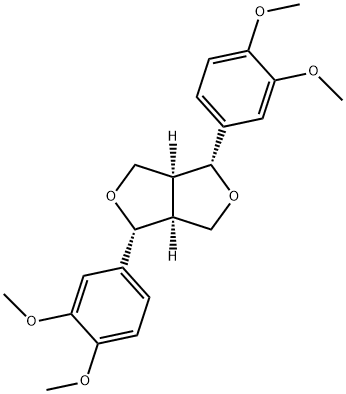
- Chemical Name:EUDESMINE
- CAS:526-06-7
- MF:C22H26O6
- Structure:
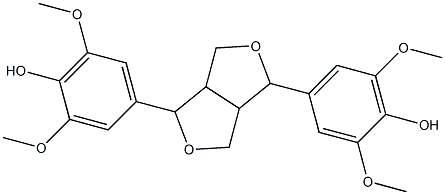
- Chemical Name:syringaresinol
- CAS:1177-14-6
- MF:C22H26O8
- Structure:
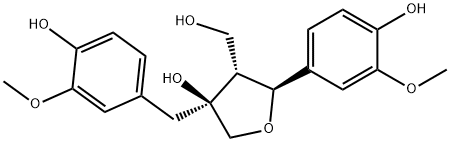
- Chemical Name:Olivil
- CAS:2955-23-9
- MF:C20H24O7
- Structure:
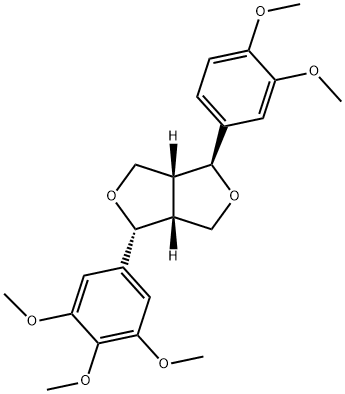
- Chemical Name:(1S,3aβ,6aβ)-1β-(3,4-Dimethoxyphenyl)-3a,4,6,6a-tetrahydro-4α-(3,4,5-trimethoxyphenyl)-1H,3H-furo[3,4-c]furan
- CAS:41689-51-4
- MF:C23H28O7
- Structure:
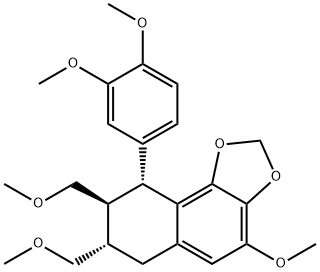
- Chemical Name:HYPOPHYLLANTHIN
- CAS:33676-00-5
- MF:C24H30O7
- Structure:
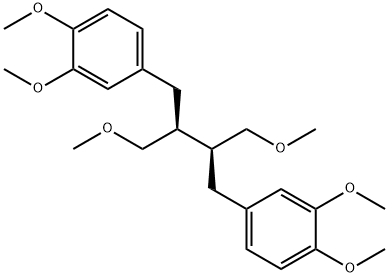
- Chemical Name:PHYLLANTHIN
- CAS:10351-88-9
- MF:C24H34O6
- Structure:
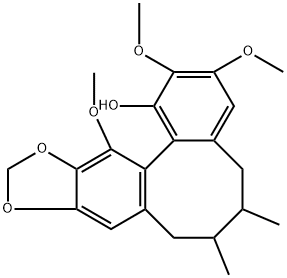
- Chemical Name:R(+)-Gomisin M1
- CAS:82467-50-3
- MF:C22H26O6
- Structure:
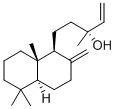
- Chemical Name:MANOOL
- CAS:596-85-0
- MF:C20H34O
- Structure:
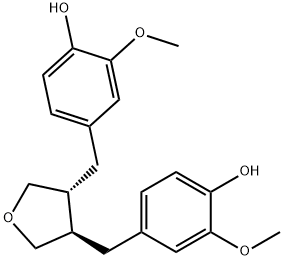
- Chemical Name:Anhydrosecoisolariciresinol
- CAS:29388-33-8
- MF:C20H24O5
- Structure:
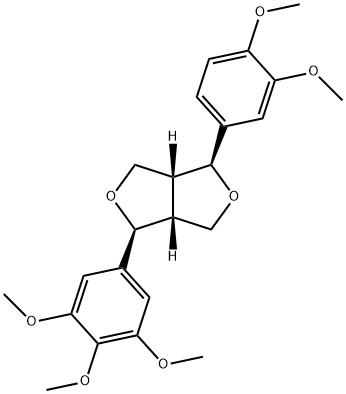
- Chemical Name:magnolin
- CAS:31008-18-1
- MF:C23H28O7
- Structure:
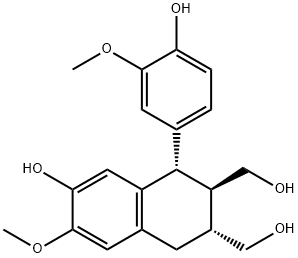
- Chemical Name:(+)-ISOLARICIRESINOL
- CAS:548-29-8
- MF:C20H24O6
- Structure:
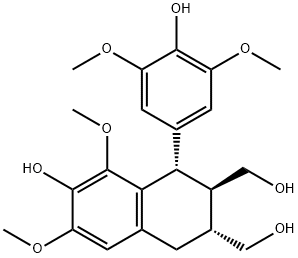
- Chemical Name:(1S)-1α-(3,5-Dimethoxy-4-hydroxyphenyl)-6,8-dimethoxy-7-hydroxy-1,2,3,4-tetrahydronaphthalene-2β,3α-dimethanol
- CAS:14464-90-5
- MF:C22H28O8
- Structure:
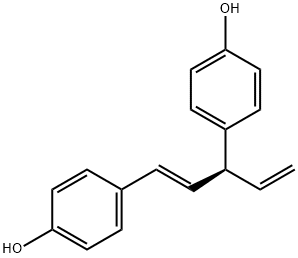
- Chemical Name:4-[(3S)-1-(4-hydroxyphenyl)penta-1,4-dien-3-yl]phenol
- CAS:17676-24-3
- MF:C17H16O2
- Structure:
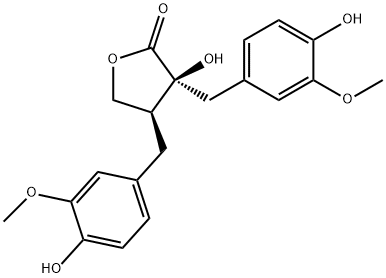
- Chemical Name:(-)-NORTRACHELOGENIN
- CAS:34444-37-6
- MF:C20H22O7
- Structure:
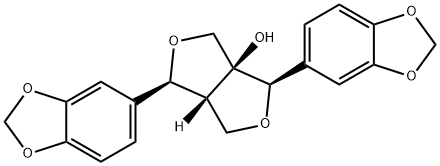
- Chemical Name:paulownin
- CAS:13040-46-5
- MF:C20H18O7
- Structure:
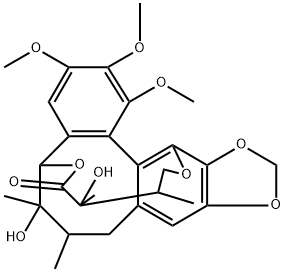
- Chemical Name:gomisin D
- CAS:60546-10-3
- MF:C28H34O10
- Structure:
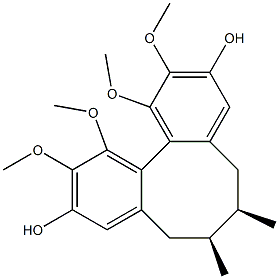
- Chemical Name:gomisin J
- CAS:66280-25-9
- MF:C22H28O6
- Structure:
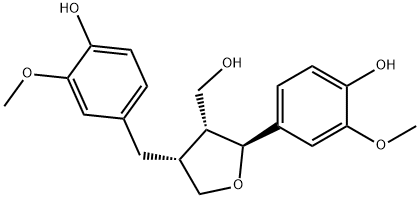
- Chemical Name:(+)-LARICIRESINOL
- CAS:27003-73-2
- MF:C20H24O6
- Structure:
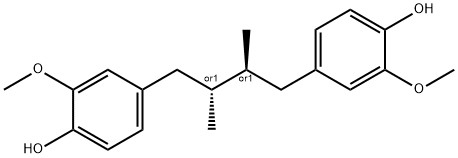
- Chemical Name:Dihydroguaiaretic acid
- CAS:66322-34-7
- MF:C20H26O4
- Structure:
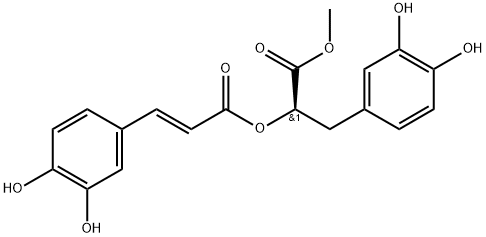
- Chemical Name:Methyl rosmarinate
- CAS:99353-00-1
- MF:C19H18O8
- Structure:
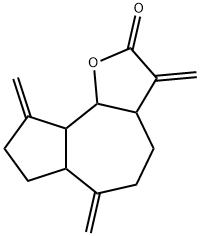
- Chemical Name:Dehydrocostuslactone
- CAS:74299-48-2
- MF:C15H18O2
- Structure:
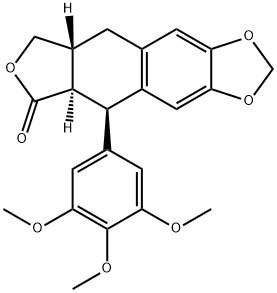
- Chemical Name:Desoxypodophyllotoxin
- CAS:19186-35-7
- MF:C22H22O7
- Structure:
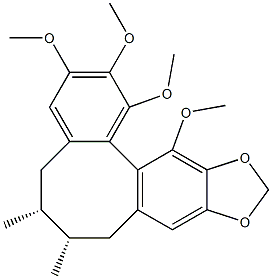
- Chemical Name:GOMISIN N
- CAS:69176-52-9
- MF:C23H28O6
- Structure:
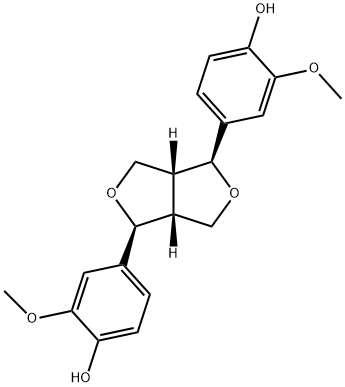
- Chemical Name:(+)-PINORESINOL
- CAS:487-36-5
- MF:C20H22O6
- Structure:
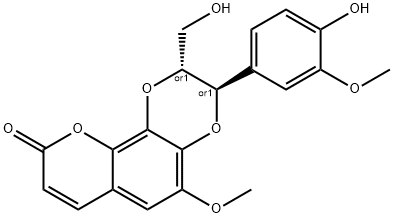
- Chemical Name:cleomiscosin A
- CAS:76948-72-6
- MF:C20H18O8
- Structure:
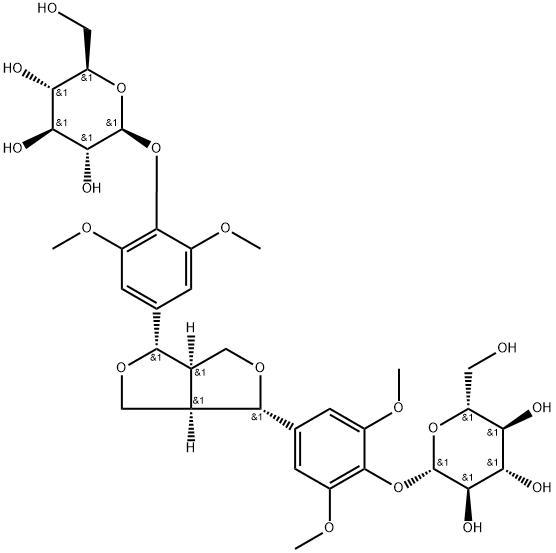
- Chemical Name:[[(3S)-3aα,4,6,6aα-Tetrahydro-1H,3H-furo[3,4-c]furan]-3α,6α-diyl]bis(2,6-dimethoxy-4,1-phenylene)bis(β-D-glucopyranoside)
- CAS:573-44-4
- MF:C34H46O18
- Structure:
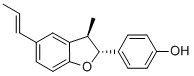
- Chemical Name:CONOCARPAN
- CAS:221666-27-9
- MF:C18H18O2
- Structure:
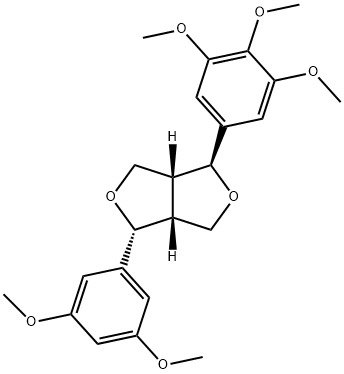
- Chemical Name:(1S,3aR,4R,6aR)-4-(3,5-Dimethoxyphenyl)tetrahydro-1-(3,4,5-trimethoxyphenyl)-1H,3H-furo[3,4-c]furan
- CAS:1134188-26-3
- MF:C23H28O7
- Structure:

- Chemical Name:PINORESINOL
- CAS:4263-88-1
- MF:C20H22O6
- Structure:
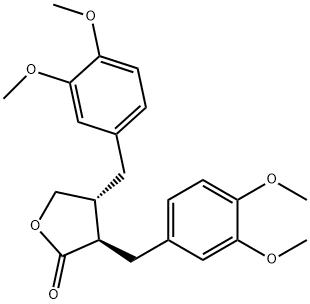
- Chemical Name:DIMETHYLMATAIRESINOL
- CAS:25488-59-9
- MF:C22H26O6
- Structure:
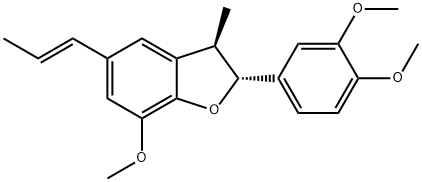
- Chemical Name:acuminatin
- CAS:41744-39-2
- MF:C21H24O4
- Structure:

- Chemical Name:(+/-)-MATAIRESINOL
- CAS:148409-36-3
- MF:C20H22O6
- Structure:
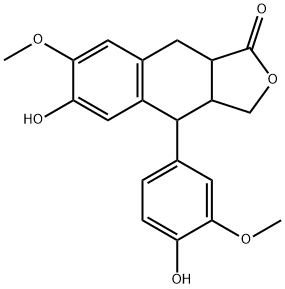
- Chemical Name:alfa-Conidendrin
- CAS:85699-62-3
- MF:C20H20O6
- Structure:
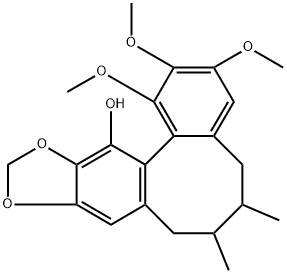
- Chemical Name:Gomisin M2
- CAS:82425-45-4
- MF:C22H26O6
- Structure:
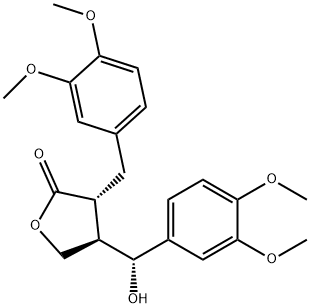
- Chemical Name:Tupichilignan A
- CAS:69586-96-5
- MF:C22H26O7
- Structure:
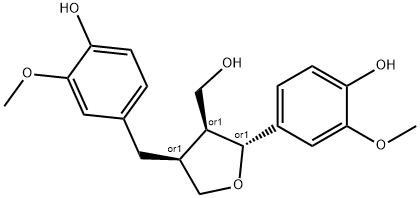
- Chemical Name:3-Furanmethanol, tetrahydro-2-(4-hydroxy-3-methoxyphenyl)-4-[(4-hydroxy-3-methoxyphenyl)methyl]-, (2R,3S,4S)-rel-
- CAS:105367-81-5
- MF:C20H24O6
- Structure:
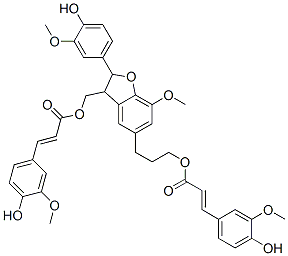
- Chemical Name:Boehmenan
- CAS:57296-22-7
- MF:C40H40O12
- Structure:
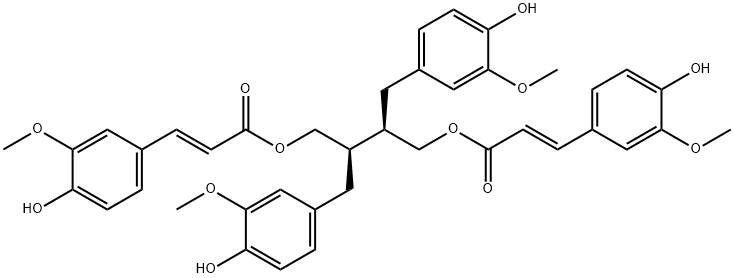
- Chemical Name:9,9'-Di-O-(E)-feruloylsecoisolariciresil
- CAS:56973-66-1
- MF:C40H42O12
- Structure:
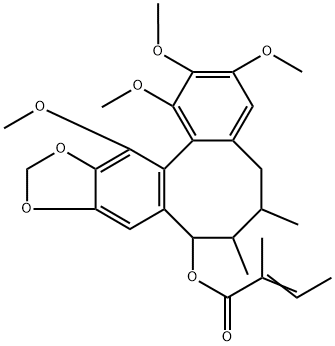
- Chemical Name:Angeloylisogomisin O
- CAS:83864-70-4
- MF:C28H34O8
- Structure:
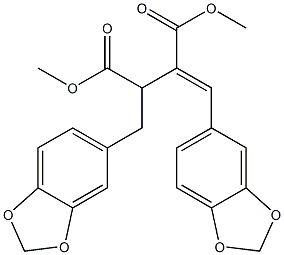
- Chemical Name:Dehydroheliobuphthalmin
- CAS:103001-05-4
- MF:C22H20O8
- Structure:
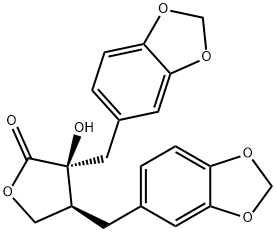
- Chemical Name:Meridil
- CAS:120051-54-9
- MF:C20H18O7
- Structure:
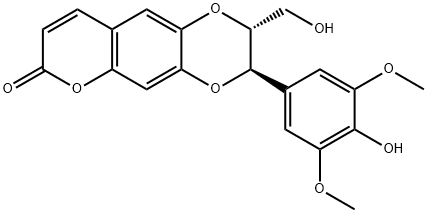
- Chemical Name:Moluccanin
- CAS:121700-26-3
- MF:C20H18O8
- Structure:
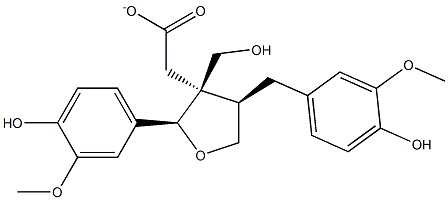
- Chemical Name:Lariciresinol acetate
- CAS:79114-77-5
- MF:C22H26O7
- Structure:
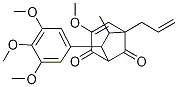
- Chemical Name:5-Allyl-3-Methoxy-6-Methyl-7-(3,4,5-tri Methoxyphenyl)bicyclo[3.2.1]oct-3-ene-2,8-dione
- CAS:106894-43-3
- MF:C22H26O6
- Structure:
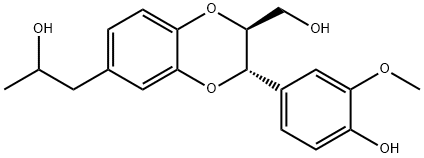
- Chemical Name:4',9,9'-Trihydroxy-3'-methoxy- 3,7'-epoxy-4,8'-oxyneolignan
- CAS:144881-21-0
- MF:C19H22O6
- Structure:
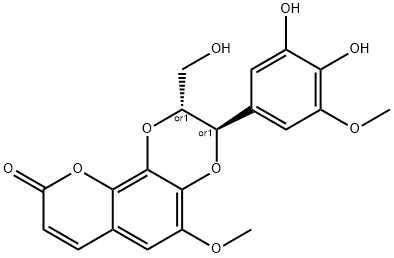
- Chemical Name:5'-Demethylaquillochin
- CAS:305364-91-4
- MF:C20H18O9
- Structure:
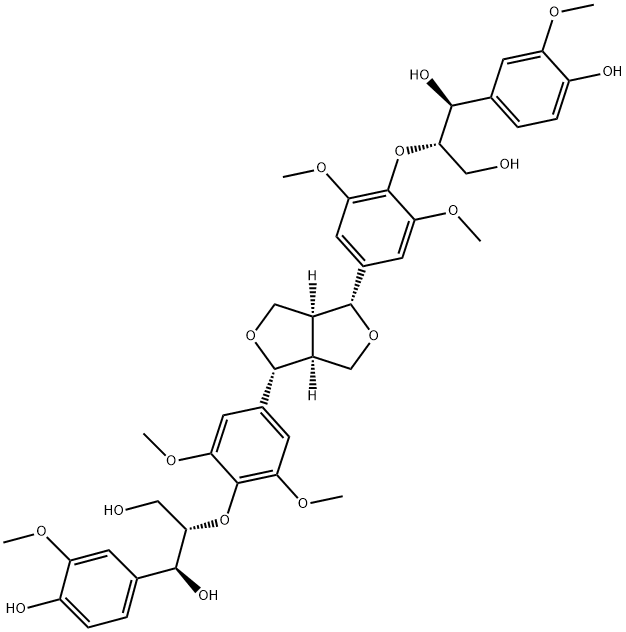
- Chemical Name:Hedyotisol A
- CAS:95732-59-5
- MF:C42H50O16
- Structure:
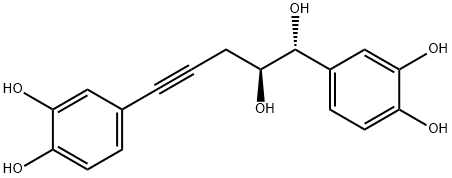
- Chemical Name:Nyasicol
- CAS:111518-95-7
- MF:C17H16O6
- Structure:
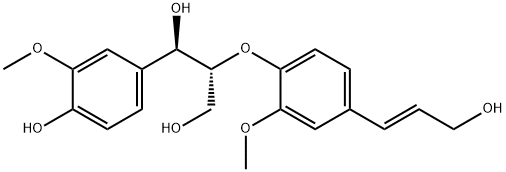
- Chemical Name:threo-Guaiacylglycerol beta-coniferyl ether
- CAS:869799-76-8
- MF:C20H24O7
- Structure:
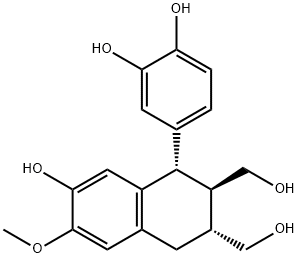
- Chemical Name:ISOTAXIRESINOL
- CAS:26194-57-0
- MF:C19H22O6
- Structure:
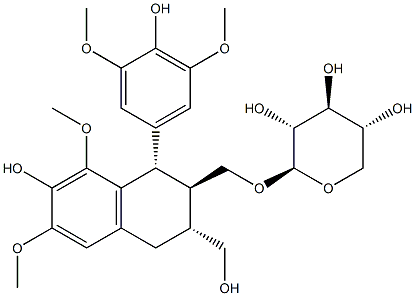
- Chemical Name:[[(1S)-1α-(3,5-Dimethoxy-4-hydroxyphenyl)-3α-(hydroxymethyl)-6,8-dimethoxy-7-hydroxytetralin-2β-yl]methyl]β-D-xylopyranoside
- CAS:34425-25-7
- MF:C27H36O12
- Structure:
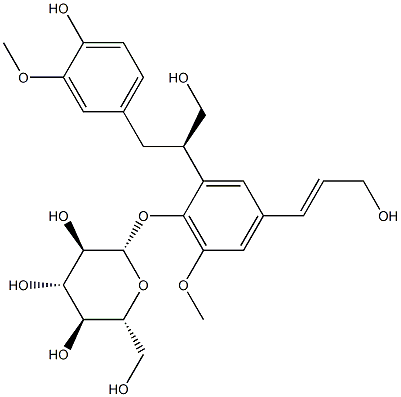
- Chemical Name:3-[4-β-D-Glucopyranosyloxy-5-methoxy-3-[2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethyl]phenyl]-2-propen-1-ol
- CAS:126176-79-2
- MF:C26H34O11
- Structure:
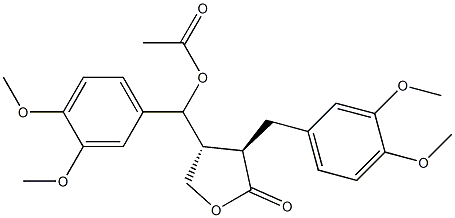
- Chemical Name:5-Acetoxymatairesil dimethyl ether
- CAS:74892-45-8
- MF:C24H28O8
- Structure:
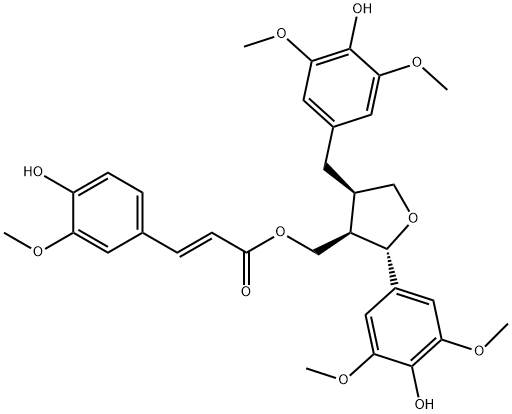
- Chemical Name:9-O-Feruloyl-5,5'-dimethoxylariciresil
- CAS:166322-14-1
- MF:C32H36O11
- Structure:
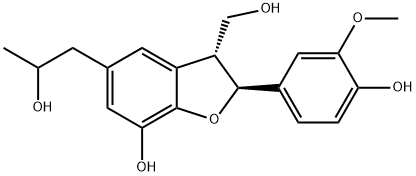
- Chemical Name:Cedrusin
- CAS:75775-36-9
- MF:C19H22O6
- Structure:
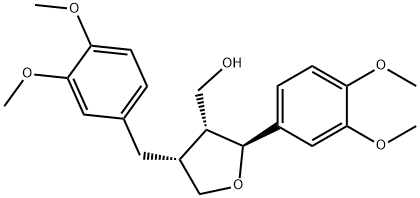
- Chemical Name:Lariciresil dimethyl ether
- CAS:67560-68-3
- MF:C22H28O6
- Structure:
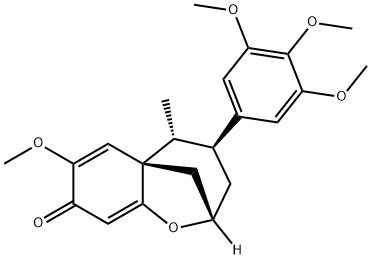
- Chemical Name:Maglifloenone
- CAS:82427-77-8
- MF:C22H26O6
- Structure:
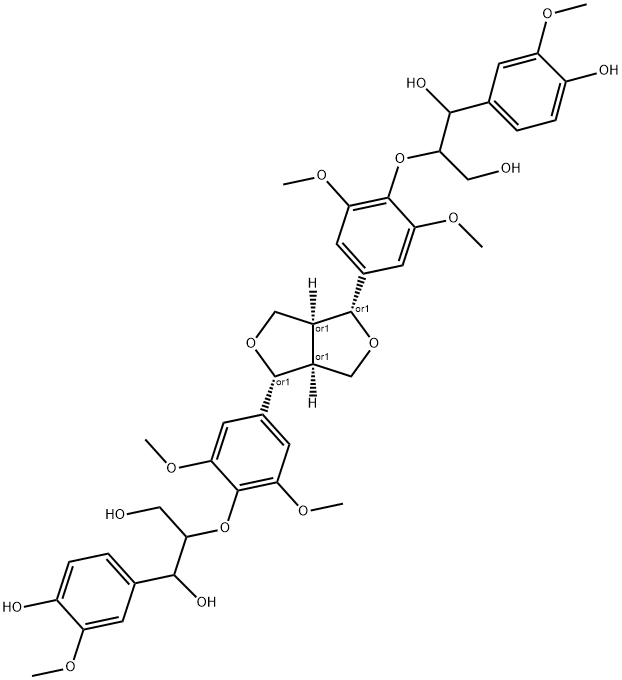
- Chemical Name:Hedyotisol B
- CAS:95839-45-5
- MF:C42H50O16
- Structure:
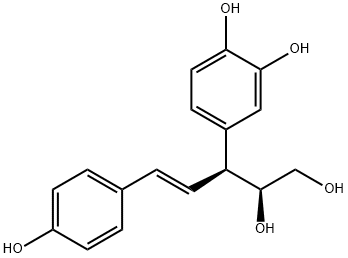
- Chemical Name:4-[(S,E)-1-[(S)-1,2-Dihydroxyethyl]-3-(4-hydroxyphenyl)-2-propenyl]-1,2-benzenediol
- CAS:18194-29-1
- MF:C17H18O5
- Structure:
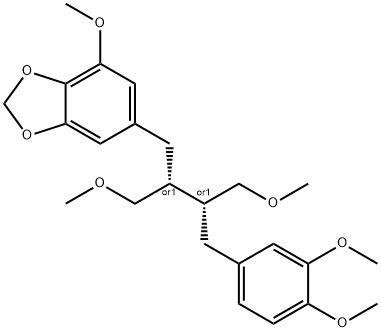
- Chemical Name:Niranthin
- CAS:50656-77-4
- MF:C24H32O7
- Structure:
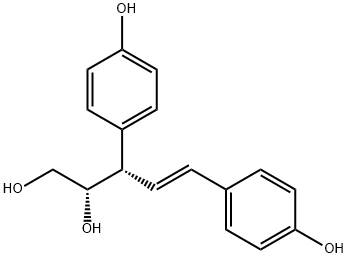
- Chemical Name:(2S,3S,4E)-3,5-Bis(4-hydroxyphenyl)-4-pentene-1,2-diol
- CAS:7288-11-1
- MF:C17H18O4
- Structure:
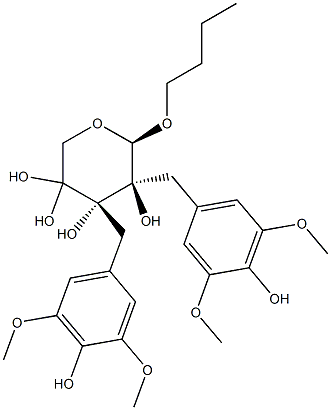
- Chemical Name:[(2R,3R)-4-(3,5-Dimethoxy-4-hydroxyphenyl)-3-(hydroxymethyl)-2-(3,5-dimethoxy-4-hydroxybenzyl)butyl]β-D-xylopyranoside
- CAS:126882-53-9
- MF:C27H38O12
- Structure:
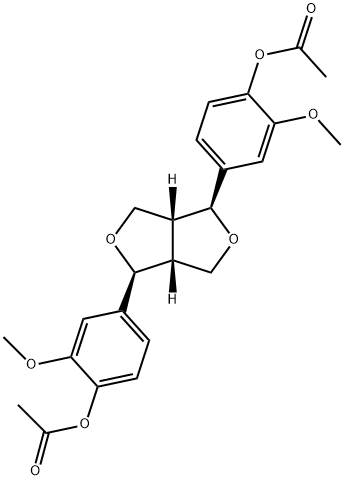
- Chemical Name:(+)-Piresil diacetate
- CAS:32971-25-8
- MF:C24H26O8
- Structure:
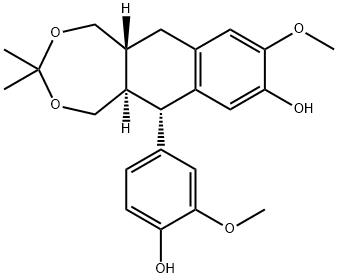
- Chemical Name:9,9'-O-isopropyllidene-isolariciresil
- CAS:252333-71-4
- MF:C23H28O6
- Structure:
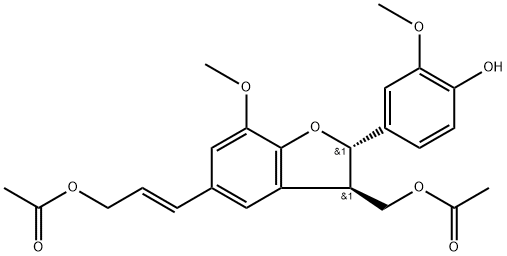
- Chemical Name:Dimeric coniferyl acetate
- CAS:184046-40-0
- MF:C24H26O8
- Structure:
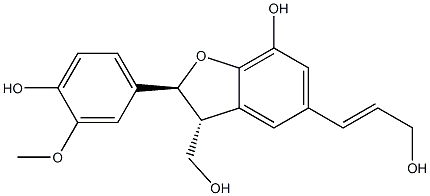
- Chemical Name:Hierochin D
- CAS:155759-02-7
- MF:C19H20O6
- Structure:
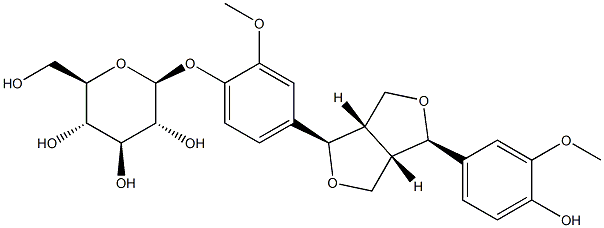
- Chemical Name:(-)-Pinoresinol 4-O-glucoside
- CAS:41607-20-9
- MF:C26H32O11
- Structure:
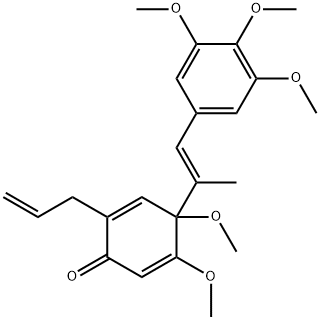
- Chemical Name:hancinone C
- CAS:111843-10-8
- MF:C23H28O6
- Structure:

- Chemical Name:(-)-PINORESINOL
- CAS:81446-29-9
- MF:C20H22O6
- Structure:
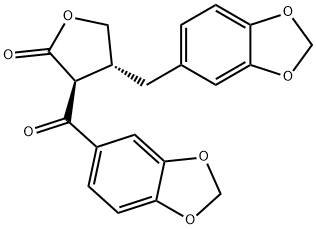
- Chemical Name:7-Oxohikinin
- CAS:130837-92-2
- MF:C20H16O7
- Structure:
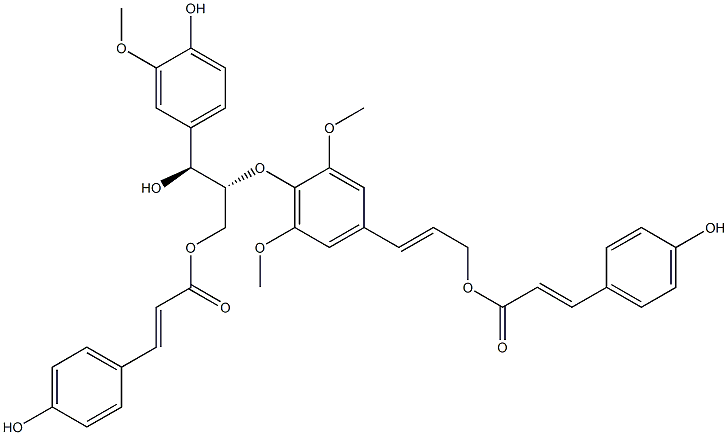
- Chemical Name:Dadahol A
- CAS:405281-76-7
- MF:C39H38O12
- Structure:
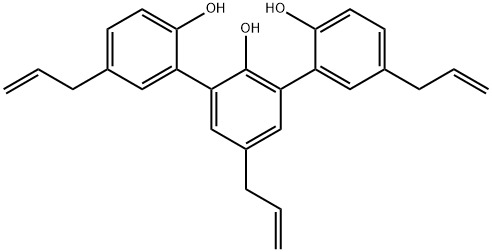
- Chemical Name:Dunnial
- CAS:139726-29-7
- MF:C27H26O3
- Structure:
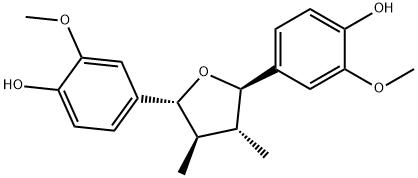
- Chemical Name:Fragransin A2
- CAS:112652-46-7
- MF:C20H24O5
- Structure:
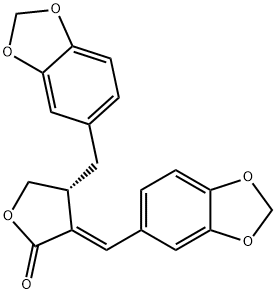
- Chemical Name:savinin
- CAS:493-95-8
- MF:C20H16O6
- Structure:
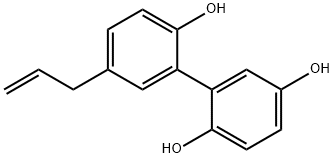
- Chemical Name:5-(2-Propenyl)-1,1'-biphenyl-2,2',5'-triol
- CAS:87562-14-9
- MF:C15H14O3
- Structure:
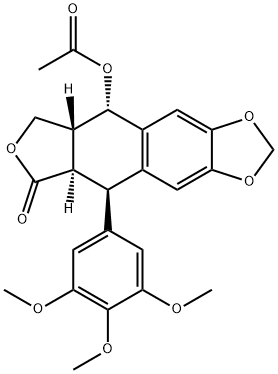
- Chemical Name:Acetylepipodophyllotoxin
- CAS:1180-35-4
- MF:C24H24O9
- Structure:
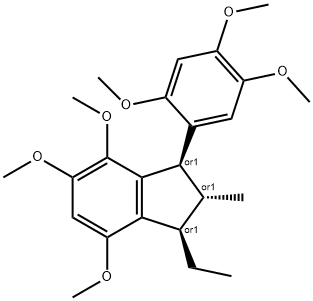
- Chemical Name:gamma-Diasarone
- CAS:80434-33-9
- MF:C24H32O6
- Structure:
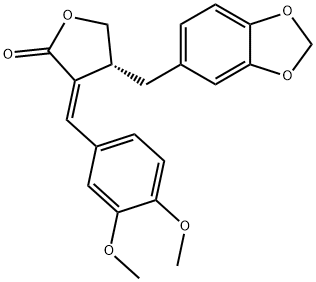
- Chemical Name:Kaerophyllin
- CAS:75590-33-9
- MF:C21H20O6
- Structure:
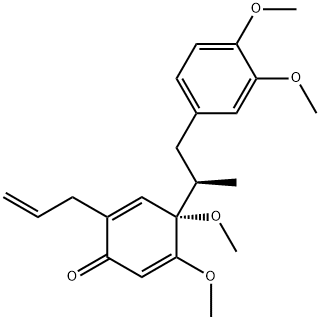
- Chemical Name:Lancifolin C
- CAS:74048-71-8
- MF:C22H28O5
- Structure:
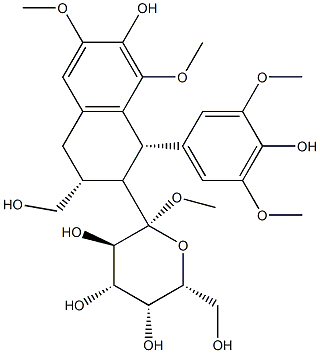
- Chemical Name:(+)-Lyoniresinol 9'-O-glucoside
- CAS:87585-32-8
- MF:C28H38O13
- Structure:
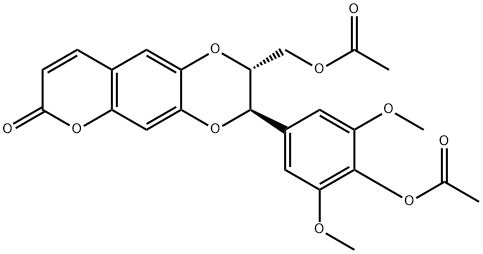
- Chemical Name:Moluccanin diacetate
- CAS:121700-27-4
- MF:C24H22O10
- Structure:
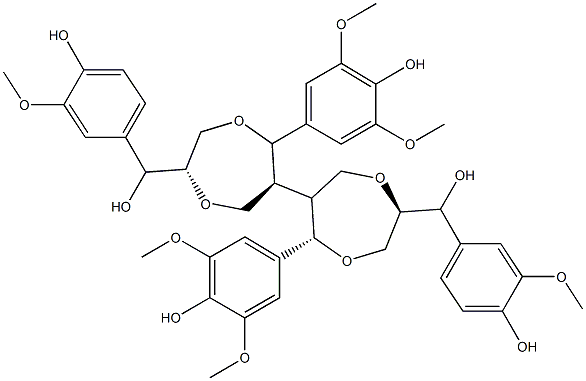
- Chemical Name:Phyllostadimer A
- CAS:638203-32-4
- MF:C42H50O16