Condensing agent
Condensing agent refers to an adjuvant which would be added in the condensation reaction. Generally there are catalytic adjuvants and catalytic adjuvants which can combine with the isolated atom or group of atoms generated during the process of condensation. About the former one, there are adjuvants such as the acid catalyst in esterification reaction and aluminum chloride in Friedl-Crafts reaction. And about the latter, there is metallic sodium in Wurtz-Fittig reaction.
A condensation reaction is a chemical reaction in which two molecules or moieties, often functional groups, combine to form a larger molecule, together with the loss of a small molecule like water, ammonia, hydrogen chloride, etc. Sometimes two organic molecules interact to form a larger molecule without emission of simple molecules. This is also known as condensation (reaction), such as aldol condensation.
The selection of condensing agents depends on the type of reaction, structure of the reactant, reaction conditions and the removed substances during the process of the reaction. Protecting groups and condensing agents in chemical synthesis: during chemical synthesis of nucleic acids, both of the nucleoside which plays the role of raw material monomers and the mononucleotide are compounds of polyfunctional group. When they are carrying chemical connections, except the groups of required reaction, other groups would also participate in the reaction, so as to generate by-products of auto-agglutination, misconnection and degradation. This will not only reduce the productivity of synthetic product, but also causes great difficulty in the separation and purification of the product. In order to ensure the phosphate diester bond structure and the nucleotide sequence, it requires a high degree of directionality and specificity in the synthetic reaction. The solution is to use a suitable protecting group and temporarily protect the group which is not required to be involved in the reaction, such as hydroxyl groups on the sugar, amino and hydroxyl phosphate on the base, etc.
Therefore, the selection of protecting groups has a great impact in whether the reaction could success or not. The selection of the condensing agents is important as well. The main reaction in the nucleic acid synthesis reaction is hydroxy reaction between phosphoric acid and RNA or DNA, which could generate phosphate ester linkage. At present, we usually use a kind of condensating agent with high activity and low side effects to react with Phosphate components and form an activated phosphate, and then accomplish the step of components condensation.
- Structure:
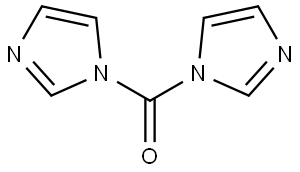
- Chemical Name:1,1'-Carbonyldiimidazole
- CAS:530-62-1
- MF:C7H6N4O
- Structure:
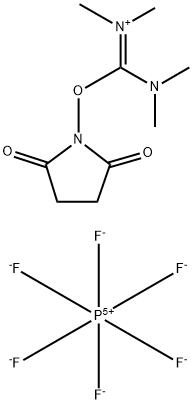
- Chemical Name:N,N,N',N'-Tetramethyl-O-(N-succinimidyl)uronium hexafluorophosphate
- CAS:265651-18-1
- MF:C9H16F6N3O3P
- Structure:
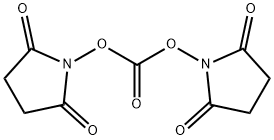
- Chemical Name:N,N'-Disuccinimidyl carbonate
- CAS:74124-79-1
- MF:C9H8N2O7
- Structure:
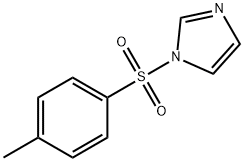
- Chemical Name:1-[(4-Methylphenyl)sulfonyl]-1H-imidazole
- CAS:2232-08-8
- MF:C10H10N2O2S
- Structure:
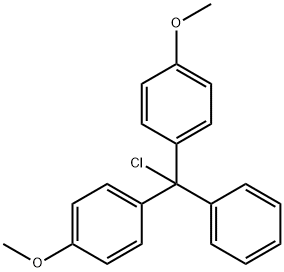
- Chemical Name:4,4'-Dimethoxytrityl chloride
- CAS:40615-36-9
- MF:C21H19ClO2
- Structure:
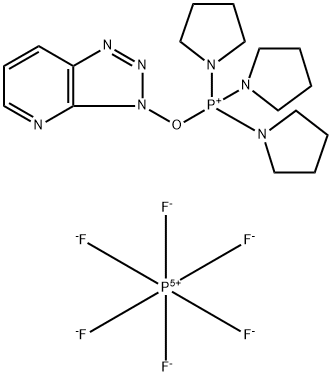
- Chemical Name:(3-Hydroxy-3H-1,2,3-triazolo[4,5-b]pyridinato-O)tri-1-pyrrolidinylphosphonium hexafluorophosphate
- CAS:156311-83-0
- MF:C17H27F6N7OP2
- Structure:
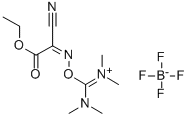
- Chemical Name:TOTU
- CAS:136849-72-4
- MF:C10H17BF4N4O3
- Structure:
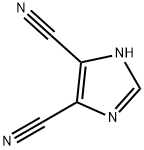
- Chemical Name:4,5-Dicyanoimidazole
- CAS:1122-28-7
- MF:C5H2N4
- Structure:
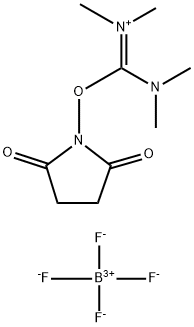
- Chemical Name:TSTU
- CAS:105832-38-0
- MF:C9H16BF4N3O3
- Structure:
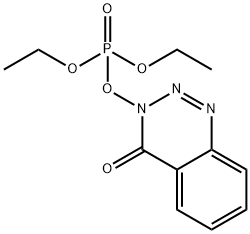
- Chemical Name:DEPBT
- CAS:165534-43-0
- MF:C11H14N3O5P
- Structure:
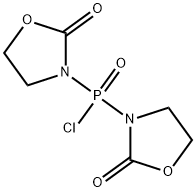
- Chemical Name:Bis(2-oxo-3-oxazolidinyl)phosphinic chloride
- CAS:68641-49-6
- MF:C6H8ClN2O5P
- Structure:
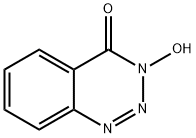
- Chemical Name:3-Hydroxy-1,2,3-benzotriazin-4(3H)-one
- CAS:28230-32-2
- MF:C7H5N3O2
- Structure:
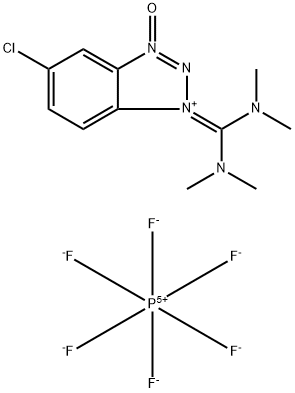
- Chemical Name:5-Chloro-1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate
- CAS:330645-87-9
- MF:C11H15ClF6N5OP
- Structure:
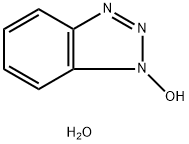
- Chemical Name:1-Hydroxybenzotriazole hydrate
- CAS:123333-53-9
- MF:C6H7N3O2
- Structure:
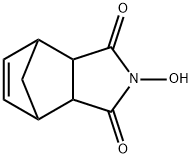
- Chemical Name:N-Hydroxy-5-norbornene-2,3-dicarboximide
- CAS:21715-90-2
- MF:C9H9NO3
- Structure:
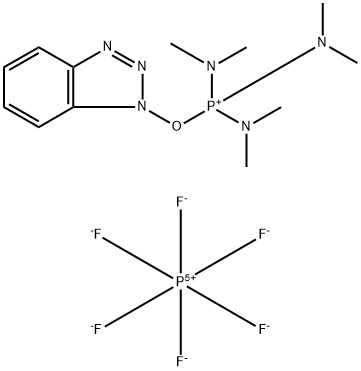
- Chemical Name:1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
- CAS:56602-33-6
- MF:C12H22F6N6OP2
- Structure:
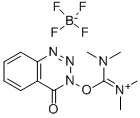
- Chemical Name:N,N,N',N'-Tetramethyl-O-(3,4-dihydro-4-oxo-1,2,3-benzotriazin-3-yl)uronium tetrafluoroborate
- CAS:125700-69-8
- MF:C12H16BF4N5O2
- Structure:
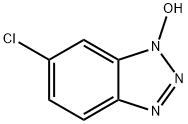
- Chemical Name:6-Chloro-1-hydroxibenzotriazol
- CAS:26198-19-6
- MF:C6H4ClN3O
- Structure:
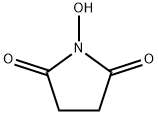
- Chemical Name:N-Hydroxysuccinimide
- CAS:6066-82-6
- MF:C4H5NO3
- Structure:
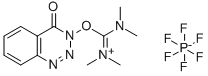
- Chemical Name:HDBTU
- CAS:164861-52-3
- MF:C12H16F6N5O2P
- Structure:
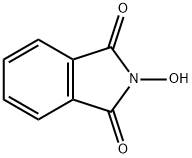
- Chemical Name:N-Hydroxyphthalimide
- CAS:524-38-9
- MF:C8H5NO3
- Structure:
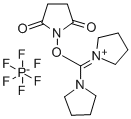
- Chemical Name:Dipyrrolidino(N-succinimidyloxy)carbenium hexafluorophosphate
- CAS:207683-26-9
- MF:C13H20F6N3O3P
- Structure:
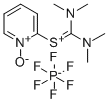
- Chemical Name:N,N,N',N'-Tetramethyl-S-(1-oxido-2-pyridyl)thiuronium hexafluorophosphate
- CAS:212333-72-7
- MF:C10H16N3OS.F6P
- Structure:
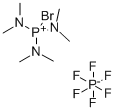
- Chemical Name:Bromotris(dimethylamino)phosphonium hexafluorophosphate
- CAS:50296-37-2
- MF:C6H18BrF6N3P2
- Structure:
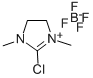
- Chemical Name:2-Chloro-1,3-dimethylimidazolidinium tetrafluoroborate
- CAS:153433-26-2
- MF:C5H10ClN2.BF4
- Structure:
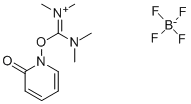
- Chemical Name:2-(2-Pyridon-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate
- CAS:125700-71-2
- MF:C10H16N3O2.BF4
- Structure:
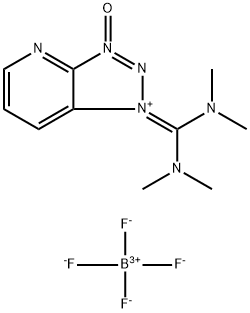
- Chemical Name:2-(7-Azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate
- CAS:873798-09-5
- MF:C10H15BF4N6O
- Structure:
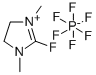
- Chemical Name:2-Fluoro-1,3-dimethylimidazolidinium hexafluorophosphate
- CAS:164298-27-5
- MF:C5H10FN2.F6P
- Structure:
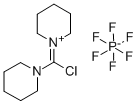
- Chemical Name:Chlorodipiperidinocarbenium hexafluorophosphate
- CAS:161308-40-3
- MF:C11H20ClF6N2P
- Structure:
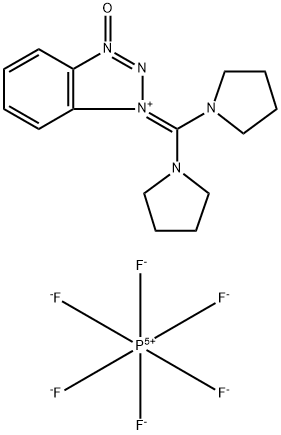
- Chemical Name:(Benzotriazol-1-yloxy)dipyrrolidinocarbenium hexafluorophosphate
- CAS:105379-24-6
- MF:C15H20F6N5OP
- Structure:
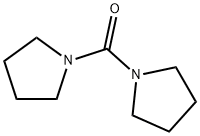
- Chemical Name:1,1'-Carbonyldipyrrolidine
- CAS:81759-25-3
- MF:C9H16N2O
- Structure:
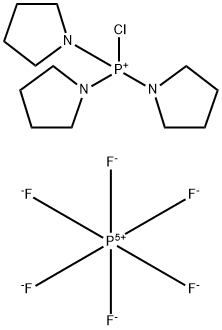
- Chemical Name:Chlorotripyrrolidinophosphonium hexafluorophosphate
- CAS:133894-48-1
- MF:C12H24ClF6N3P2
- Structure:
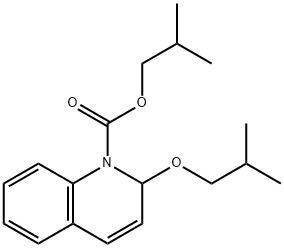
- Chemical Name:Isobutyl 1,2-dihydro-2-isobutoxy-1-quinoline-carboxylate
- CAS:38428-14-7
- MF:C18H25NO3
- Structure:
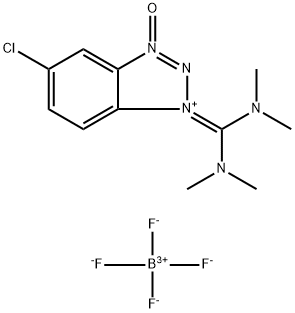
- Chemical Name:O-(6-Chlorobenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate
- CAS:330641-16-2
- MF:C11H15BClF4N5O
- Structure:
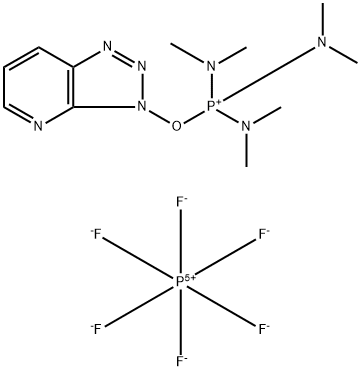
- Chemical Name:7-Azabenzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate
- CAS:156311-85-2
- MF:C11H21F6N7OP2
- Structure:
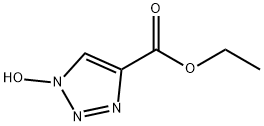
- Chemical Name:Ethyl 1-hydroxy-1H-1,2,3-triazole-4-carboxylate
- CAS:137156-41-3
- MF:C5H7N3O3
- Structure:
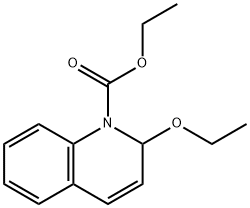
- Chemical Name:N-Ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline
- CAS:16357-59-8
- MF:C14H17NO3
- Structure:
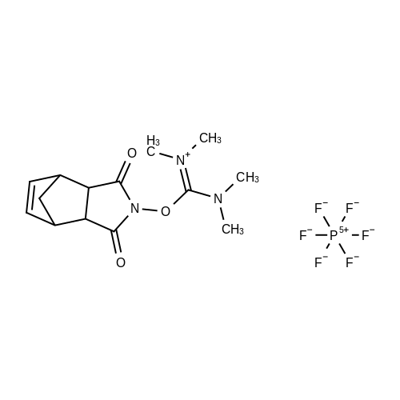
- Chemical Name:HNTU
- CAS:208462-94-6
- MF:C14H20F6N3O3P
- Structure:
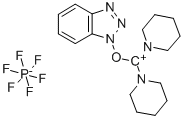
- Chemical Name:(Benzotriazol-1-yloxy)dipiperidinocarbenium hexafluorophosphate
- CAS:190849-64-0
- MF:C17H24F6N5OP
- Structure:
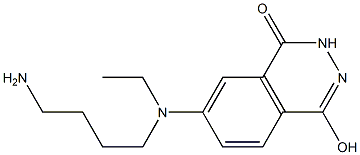
- Chemical Name:N-(4-Aminobutyl)-N-ethylisoluminol
- CAS:66612-29-1
- MF:C14H20N4O2
- Structure:
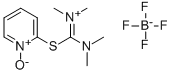
- Chemical Name:2-(1-Oxy-pyridin-2-yl)-1,1,3,3-tetramethylisothiouronium tetrafluoroborate
- CAS:255825-38-8
- MF:C10H17BF4N3OS*