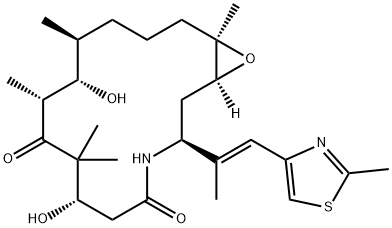
Ixabepilone, a semisynthetic analog of epothilone B, was launched for the treatment of metastatic or locally advanced breast cancer. It is indicated
for use in combination with capecitabine in patients who have previously failed
treatment with an anthracycline such as doxorubicin and a taxane such as
paclitaxel. It is also approved as monotherapy for the treatment of metastatic or
locally advanced breast cancer in patients whose tumors are resistant or
refractory to anthracyclines, taxanes, and capecitabine. Ixabepilone is the first
member of the epothilone family of anticancer agents to be approved.
Epothilones are novel cytotoxic macrolides derived from bacterial fermentation.
Like the taxanes, their mechanism of action involves binding to and stabilizing microtubules, which results in mitotic arrest and apoptosis.
The most common adverse reactions (X20%) associated with ixabelipone as monotherapy or in combination with capecitabine were peripheral sensory neuropathy, fatigue/asthenia, myalgia/arthralgia, alopecia, nausea, vomiting, stomatitis/ mucositis, diarrhea, and musculoskeletal pain. The most common hematologic abnormalities (W 40%) include neutropenia, leukopenia, anemia, and thrombocytopenia. Ixabelipone in combination with capecitabine is contraindicated in patients with AST or ALT W2.5 ULN (upper limit of normal) or bilirubin W1 ULN due to increased risk of toxicity and neutropenia-related death.