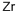General Description
A gray amorphous sludge with not less than 20% water.
Reactivity Profile
When a mixture of alkali hydroxides and zirconium is heated, the liberated oxygen reacts explosively with zirconium [Mellor 7:116 1946-47]. Chromates, dichromates, sulfates, molybdates, and tungstates of lithium, sodium, potassium, rubdium, and cesium will react violently, even explosively, with an excess of zirconium powder [Ellern 1968. p. 249]. A mixture of hydrated borax and zirconium explodes when heated [Mellor 7:116 1946-47]. An explosion occurred when zirconium sponge was placed in a beaker of carbon tetrachloride [Allison 1969]. Zirconium explodes violently with cupric oxide or lead oxide [Mellor 7:116 1946-47]. A mixture of powdered zirconium and potassium nitrate explodes when heated above the melting point [Mellor 7:116 1946-47].
Air & Water Reactions
May ignite on contact with air or moist air. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. The severity of the pyrophoric reaction depends a great deal on zirconium particle size, with the finely divided material reacting with the most vigor. The initiation of the explosion has been spark and by electrostatic ignition. Zirconium dusts have been known to explode, [NFPA 482M, 1974], covers all aspects of storage and handling of zirconium, there are 43 abstracts of unusual zirconium fire and explosion incidents. Water Insoluble .
Potential Exposure
Zirconium is never found in the free
state; the most common sources are the ores zircon and
baddeleyite. It is generally produced by reduction of the
chloride or iodide. The metal is highly reactive; the process
is usually performed under an inert gas blanket. Zirconium
metal is used as a “getter” in vacuum tubes, a deoxidizer in
metallurgy; a substitute for platinum; it is used in priming
of explosive mixtures; flashlight powders; lamp filaments;
flash bulbs; and construction of rayon spinnerets.
Zirconium or its alloys (with nickel, cobalt, niobium, tantalum)
are used as lining materials for pumps and pipes, for
chemical processes, and for reaction vessels. Pure zirconium
is a structural material for atomic reactor; and
alloyed, particularly with aluminum, it is a cladding material
for fuel rods in water-moderated nuclear reactors. A
zirconium-columbium alloy is an excellent superconductor.
Zircon (ZrSiO4) is utilized as a foundry sand, an abrasive;
a refractory in combination with zirconia; a coating for
casting molds; a catalyst in alkyl and alkenyl hydrocarbon
manufacture; a stabilizer in silicone rubbers; and as a gem
stone; in ceramics it is used as an opacifier for glazes and
enamels and in fritted glass filters. Both zircon and zirconia
(zirconium oxide, ZrO2) bricks are used as linings for glass
furnaces. Zirconia itself is used in die extrusion of metals
and in spout linings for pouring metals, as a substitute for
lime in oxyhydrogen flam; as a pigment; and an abrasive; it
is used, too, in incandescent lights; as well as in the manufacture
of enamels, white glass; and refractory crucibles.
Other zirconium compounds are used in metal cutting tools,
thermocouple jackets; waterproofing textiles; ceramics, and
in treating dermatitis and poison ivy.
Fire Hazard
May react violently or explosively on contact with water. Some are transported in flammable liquids. May be ignited by friction, heat, sparks or flames. Some of these materials will burn with intense heat. Dusts or fumes may form explosive mixtures in air. Containers may explode when heated. May re-ignite after fire is extinguished.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN2008 Zirconium powder, dry, Hazard Class:
4.2; Labels: 4.2-Spontaneously combustible material.
UN1358 Zirconium suspended in a liquid, Hazard Class: 3;
Labels: 3-Flammable liquid. UN1358 Zirconium powder,
wetted with not <25% water (a visible excess of water
must be present) (1) mechanically produced, particle size
<53 μm; (2) chemically produced, particle size <840 μm,
Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN 1932
Zirconium scrap, Hazard Class: 4.2; Labels: 4.2-
Spontaneously combustible material. UN 2009 Zirconium,
dry, finished sheets, strip or coiled wire, Hazard Class: 4.2;
Labels: 4.2-Spontaneously combustible material. UN2858
Zirconium, dry, coiled wire, finished metal sheets, strip
(thinner than 254 μm but not thinner than 18 μm), Hazard
Class: 4.1; Labels: 4.1-Flammable solid.
Incompatibilities
Dust may form explosive mixture with
air. Violent reactions with oxidizers, air, alkali hydroxides;
alkali metal compounds (such as chromates, dichromates,
molybdates, salts; sulfates, and tungstates); borax, carbon
tetrachloride; lead, lead oxide; phosphorus, potassium compound
s. Incompatible with boron, carbon, nitrogen, halogens,
lead, platinum, potassium nitrate. Powder may ignite
spontaneously and can continue burning under water.
Explodes if mixed with hydrated borax when heated. Fine
powder may be stored completely immersed in water.
Description
Zirconium, is a metallic element with a grayish, crystalline scale or gray amorphous powder form. It is flammable or explosive in the form of a powder or dust and as borings and shavings. The powder should be kept wet in storage. Zirconium is a suspected carcinogen, with a TLV of 5 mg/m3 of air and it is insoluble in water. The four-digit UN identification numbers depend on the form and the amount of water present. The number for zirconium, dry, as wire, sheeting, or in the form of strips is 2009. The number for zirconium, dry, as a wire, sheeting, or as strips that are thinner than 254 μm, but not thinner than 18 μm, is 2858. Dry zirconium metal powder is 2008. Wet zirconium powder is 1358. Zirconium metal in a liquid suspension is 1308. The primary uses are as a coating on nuclear fuel rods, photo flashbulbs, pyrotechnics, explosive primers, and laboratory crucibles.
Chemical Properties
Zirconium is a grayish-white, lustrous metal in the form of platelets, flakes, or a bluish-black, amorphous powder. It has a negligible vapor pressure, and is insoluble in water, especially as zirconium oxide. The primary valence state is 4+. Zirconium and its alloys react violently with strong acids and are incompatible with strong metal alkalis and strong oxidizers but are inert to most weak acids and alkalis. Pure zirconium powder may explode spontaneously in air, while zirconium hydrides react with water to produce a flammable gas. Zirconium tetrachloride is highly corrosive, which hydrolyzes in water to form zirconyl chloride.

Physical properties
Zirconium can be a shiny grayish crystal-like hard metal that is strong, ductile, and malleable,or it can be produced as an undifferentiated powder. It is reactive in its pure form.Therefore, it is only found in compounds combined with other elements—mostly oxygen.Zirconium-40 has many of the same properties and characteristics as does hafnium-72, whichis located just below zirconium in group 4 of the periodic table. In fact, they are more similarthan any other pairs of elements in that their ions have the same charge (+4) and are of thesame general size. Because zirconium is more abundant and its chemistry is better knownthan hafnium’s, scientists extrapolate zirconium’s properties for information about hafnium.This also means that one “twin” contaminates the other, and this makes them difficult toseparate.
Zirconium’s melting point is 1,852°C, its boiling point is 4,377°C, and its density is 6.506g/cm3.
Isotopes
Zirconium has 37 isotopes, ranging from Zr-79 to Zr-110. Four of them arestable, and one is a naturally radioactive isotope, with a very long half-life. All five contribute to the element’s natural existence on Earth. The stable isotopes are the following:Zr-90 = 1.45%, Zr-91 = 11.22%, Zr-92 = 17.15%, and Zr-94 = 17.38%. The one naturalradioactive isotope is considered stable: Zr-96, with a half-life of 2.2 × 10+19 years,contributes 2.80% to zirconium’s total existence on Earth. All of the other isotopes are artificially radioactive and are produced in nuclear reactors or particle accelerators. They have half-lives ranging from 150 nanoseconds to 1.53 × 10+6 years.
Origin of Name
The name “zirconium” was derived from the Arabic word zargun, which
means “gold color.” Known in biblical times, zirconium mineral had several names (e.g.,
jargoon, jacith, and hyacinth). Later, the mineral was called “zirconia,” and the element
was later named “zirconium.”
Occurrence
Zirconium is not a rare element. It is found over most of Earth’s crust and is the 18th mostabundant element, but it is not found as a free metal in nature.
It is found in the ores baddeleyite (also known as zirconia) and in the oxides of zircons,elpidite, and eudialyte.
Characteristics
Zirconium is insoluble in water and cold acids. Although it is a reactive element, it resistscorrosion because of its rapid reaction with oxygen, which produces a protective film of zirconiumoxide (ZrO2) that protects any metal with which it is coated. Zirconium is best knownas the gemstone zircon. Although there are different types of zircons, the most recognized isthe hard, clear, transparent zircon crystal that has a very high index of refraction, which meansit can bend light at great angles. These zircon crystals (zirconium sulfate, ZrSiO4) are cut withfacets to resemble diamonds.
Another characteristic that makes zirconium useful is the production of “zircaloy,” whichdoes not absorb neutrons as does stainless steel in nuclear reactors. Thus, it is ideal to makenuclear fuel tubes and reactor containers. Zircaloy is the blend (alloy) of zirconium and anyof several corrosion resistant metals.
Production Methods
Zirconium was first produced in elemental form in 1824 by
Berzelius, but it was brittle because it contained impurities
such as oxygen, nitrogen, and hydrogen. In 1914, the first
relatively pure zirconium was prepared by reducing zirconium
tetrachloride with sodium in a bomb furnace. Highpurity
zirconium was produced by Van Arkel and de Boer in
1925 by vaporizing zirconium tetraiodide into a bulb containing
a hot tungsten filament, which caused the tetraiodide to
dissociate, depositing zirconium on the filament. The zirconiumisrecoveredas
brightextremelypuremetalcrystals.This
procedure was later used for the commercial production of
zirconium in the United States. Dr. Kroll from the Bureau of
Mines conducted research and produced high-purity zirconium
on a commercial scale in 1944; the Kroll method is now
used for large-scale commercial production of zirconium.
In 2009, resources of zircon in the United States included
about 14 million tons associated with titanium resources in
heavy-mineral sand deposits. Phosphate and sand and
gravel deposits have the potential to yield substantial amount
of zircon as a future by-product. Eudialyte and gittinsite are
zirconium silicate minerals that have a potential for zirconia
production. Currently, identified world resources of zircon
exceed 60 million tons.
The production of zirconium metal must be carried out in
an atmosphere from which water vapor, oxygen, and nitrogen are rigorously excluded; otherwise, the metal becomes brittle
and impossible to fabricate.
Hazard
There is disagreement relative to the dangers of the elemental form of zirconium. Some saythat the metal and gemstone forms are harmless, but there is some evidence that the vapors andpowder forms of the metal may be carcinogenic. Also, several zirconium compounds can produceallergic reactions in humans and have proven to be toxic to the skin or lungs if inhaled.
The fine powder and dust of zirconium are explosive, especially in the presence of nonmetalsthat oxidize these forms of zirconium.
Health Hazard
The toxicity of zirconium and its compoundshas been found to be of low order. Lethal dosein rabbits when administered intravenouslyis reported as 150 mg/kg (Lewis(Sr) 1996).Inhalation of dust of the metal or its compoundscan form skin and pulmonary granulomasthat may be attributed to reaction ofsensitized T cells with antigen. X-ray studiesin animals indicate retention of the metal inthe lungs. Inhalation may produce irritationof mucous membrane. Skin contact can causeirritation.
Flammability and Explosibility
Contactwithwaterliberateshighlyflammablegases